Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (11): 1360-1371.DOI: 10.6023/A21070340 Previous Articles Next Articles
Review
投稿日期:2021-07-23
发布日期:2021-08-23
通讯作者:
奚江波, 陈伟
作者简介: |
黄杰, 2019年本科毕业于武汉工程大学, 目前在武汉工程大学化学与环境工程学院柏正武课题组开展研究工作, 研究方向是碳基催化剂在有机反应中的应用. |
 |
奚江波, 博士、副教授、硕士生导师. 主要研究领域涉及高性能贵金属纳米催化剂、单原子催化剂和无金属碳基催化剂的制备及其在有机催化、电催化、生物传感等方面的应用, 以及催化机理的研究. 近年来在Advanced Functional Materials、Applied Catalysis B: Environmental、Journal of Catalysis等国际期刊上发表了学术论文30余篇, 获授权中国发明专利5项. |
 |
陈伟, 博士, 副教授, 硕士生导师. 主要研究领域涉及无机材料的合成、表征与应用, 并从事手性分离材料的研究及色谱分析工作. 主持湖北省教育厅科学技术研究计划重点项目1项, 在国内外学术期刊上发表科研论文20余篇, 其中SCI收录论文7篇, 授权发明专利1项. |
 |
柏正武, 博士、教授、博士生导师. 研究领域涉及有机合成、药物研制及手性功能材料的制备与应用. 主持并完成了4项国家自然科学基金资助的面上项目, 取得了一些有自主知识产权的研究成果, 获得多项发明专利权, 在国际学术期刊上发表研究论文60余篇. |
基金资助:
Jie Huang, Jiangbo Xi( ), Wei Chen(
), Wei Chen( ), Zhengwu Bai
), Zhengwu Bai
Received:2021-07-23
Published:2021-08-23
Contact:
Jiangbo Xi, Wei Chen
Supported by:Share
Jie Huang, Jiangbo Xi, Wei Chen, Zhengwu Bai. Graphene-derived Materials for Metal-free Carbocatalysis of Organic Reactions[J]. Acta Chimica Sinica, 2021, 79(11): 1360-1371.
| Catalyst | Preparation method | Functional groups or dopants | Reaction |
|---|---|---|---|
| GO[ | Hummers method | O-containing groups | Oxidation of alcohol |
| abGO[ | GO was treated with sequential base and acid | O-containing groups | |
| NG-T[ | GO annealed under NH3 flow | Doped N atoms | |
| BG[ | GO annealed with boric acid | Doped B atoms (2.9%) | |
| G1000[ | Pyrolysis of the sulfate lignin | Doped S and O atoms | |
| CCG[ | Chemical reduction of GO | π-system of graphene | Oxidation of benzene |
| GO[ | Modified Hummers method | O-containing groups | Oxidation of cyclohexane |
| NGO[ | Modified Hummers method | O-containing groups | Oxidation of alkyl-substituted arenes |
| LC-N[ | CVD method with acetonitrile as N source | Doped N atoms (8.9%) | |
| NG[ | Arc-discharge exfoliation and subsequently annealed with NH3 | Doped N atoms (0.42%) | Oxidation of olefin |
| rGO[ | GO reduction (modified Wallace's method) | The zigzag edges of rGO | Reduction of nitroaromatics |
| NG[ | Hydrothermal treatment of GO-urea mixture and subsequent annealing | Doped N atoms (2.03%) | |
| NHG[ | Hydrothermal treatment of GO-NH3-H2O2 mixture | Doped N atoms(9.77%) | |
| NPG[ | Pyrolysis of a microwave-exfoliated graphite and hexachlorocyclotriphosphazene | Doped N and P atoms | |
| Graphene[ | Pyrolysis of alginate | Lewis acid-base pairs | Selective acetylene hydrogenation |
| GO[ | Modified Hummers method | O-containing groups | Cross-coupling reaction |
| GO[ | — | O-containing groups | |
| Graphite oxide[ | Modified Hummers method | O-containing groups | Oxidative coupling of amines |
| ba-GO[ | GO was treated with base and acid | O-containing groups | |
| PG[ | Annealing of triphenylphosphine | Doped P atoms | |
| BNHG[ | Annealing of of dicyandiamide, glucose and boric acid | Doped B and N atoms | |
| GO[ | Modified Hummers method | O-containing groups | Friedel-Crafts alkylation |
| GO[ | Modified Hummers method | O-containing groups | |
| GO[ | Hummers method | O-containing groups | |
| Graphite[ | — | — | Friedel-Crafts acylation |
| GR-SO3H[ | rGO reacts with sodium nitrite and sulfanilic acid at room temperature | Denzenesulfonic acid groups | Transesterification |
| GO-S[ | Modified Hummers method | Sulfonic acid groups and O-containing groups | Esterification |
| Graphite oxide[ | Hummers method | — | Claisen-Schmidt condensation |
| GO-DETA[ | GO modification with triethylamine | Primary, secondary amino and Oxygen-containing groups | Michael reaction and Knoevenagel reaction |
| GO[ | Modified Hummers method | Oxygen-containing groups | Hydrolysis reaction |
| GO[ | — | O-containing groups | N-formylation reaction |
| GO[ | — | O-containing groups | Dehydrogenation reaction |
| GO[ | — | O-containing groups | Iodization reaction |
| Graphite oxide[ | Hummers method | O-containing groups | Dehydration reaction |
| Graphite[ | — | — |
| Catalyst | Preparation method | Functional groups or dopants | Reaction |
|---|---|---|---|
| GO[ | Hummers method | O-containing groups | Oxidation of alcohol |
| abGO[ | GO was treated with sequential base and acid | O-containing groups | |
| NG-T[ | GO annealed under NH3 flow | Doped N atoms | |
| BG[ | GO annealed with boric acid | Doped B atoms (2.9%) | |
| G1000[ | Pyrolysis of the sulfate lignin | Doped S and O atoms | |
| CCG[ | Chemical reduction of GO | π-system of graphene | Oxidation of benzene |
| GO[ | Modified Hummers method | O-containing groups | Oxidation of cyclohexane |
| NGO[ | Modified Hummers method | O-containing groups | Oxidation of alkyl-substituted arenes |
| LC-N[ | CVD method with acetonitrile as N source | Doped N atoms (8.9%) | |
| NG[ | Arc-discharge exfoliation and subsequently annealed with NH3 | Doped N atoms (0.42%) | Oxidation of olefin |
| rGO[ | GO reduction (modified Wallace's method) | The zigzag edges of rGO | Reduction of nitroaromatics |
| NG[ | Hydrothermal treatment of GO-urea mixture and subsequent annealing | Doped N atoms (2.03%) | |
| NHG[ | Hydrothermal treatment of GO-NH3-H2O2 mixture | Doped N atoms(9.77%) | |
| NPG[ | Pyrolysis of a microwave-exfoliated graphite and hexachlorocyclotriphosphazene | Doped N and P atoms | |
| Graphene[ | Pyrolysis of alginate | Lewis acid-base pairs | Selective acetylene hydrogenation |
| GO[ | Modified Hummers method | O-containing groups | Cross-coupling reaction |
| GO[ | — | O-containing groups | |
| Graphite oxide[ | Modified Hummers method | O-containing groups | Oxidative coupling of amines |
| ba-GO[ | GO was treated with base and acid | O-containing groups | |
| PG[ | Annealing of triphenylphosphine | Doped P atoms | |
| BNHG[ | Annealing of of dicyandiamide, glucose and boric acid | Doped B and N atoms | |
| GO[ | Modified Hummers method | O-containing groups | Friedel-Crafts alkylation |
| GO[ | Modified Hummers method | O-containing groups | |
| GO[ | Hummers method | O-containing groups | |
| Graphite[ | — | — | Friedel-Crafts acylation |
| GR-SO3H[ | rGO reacts with sodium nitrite and sulfanilic acid at room temperature | Denzenesulfonic acid groups | Transesterification |
| GO-S[ | Modified Hummers method | Sulfonic acid groups and O-containing groups | Esterification |
| Graphite oxide[ | Hummers method | — | Claisen-Schmidt condensation |
| GO-DETA[ | GO modification with triethylamine | Primary, secondary amino and Oxygen-containing groups | Michael reaction and Knoevenagel reaction |
| GO[ | Modified Hummers method | Oxygen-containing groups | Hydrolysis reaction |
| GO[ | — | O-containing groups | N-formylation reaction |
| GO[ | — | O-containing groups | Dehydrogenation reaction |
| GO[ | — | O-containing groups | Iodization reaction |
| Graphite oxide[ | Hummers method | O-containing groups | Dehydration reaction |
| Graphite[ | — | — |
| Reaction | Catalyst | Substrate | Product | Probable active site |
|---|---|---|---|---|
| Oxidation of alcohol | GO[ | Alcohol | Ketone/aldehyde | Epoxide groups |
| abGO[ | Benzylic alcohol | Benzaldehyde | Phenol hydroxyl groups | |
| NG-T[ | Benzylic alcohol | Benzaldehyde | Graphitic sp2-N sites | |
| BG[ | Benzylic alcohol | Benzaldehyde | B doping species (BC3) | |
| G1000[ | Benzylic alcohol | Benzaldehyde | Doped S and O atoms | |
| Oxidation of benzene | CCG[ | Benzene | Phenol | π-system of graphene and O-containing groups |
| Oxidation of cyclohexane | GO[ | Cyclohexane | Cyclohexanol Cyclohexanone Adipic acid | Carboxylic acid groups |
| Oxidation of alkyl- substituted arenes | NGO[ | Toluene | Benzoic acid | — |
| LC-N[ | Ethylbenzene | Acetophenone | C atoms adjacent to N atoms | |
| Oxidation of olefin | NG[ | Olefin | Alkylene oxide | Graphite N |
| Reduction of nitroaromatics | rGO[ | Nitrobenzene | Aniline | Edge sites |
| NG[ | Nitroaromatics | Aromatic amine | Graphitic N | |
| Reaction | Catalyst | Substrate | Product | Probable active site |
| NHG[ | Nitroaromatics | Aromatic amine | Doped N atoms | |
| NPG[ | Nitroaromatics | Aromatic amine | Doped N and P atoms | |
| Selective hydrogenation | Graphene[ | Acetylene Ethylene | Ethylene Ethane | Lewis acid-base pairs |
| Cross-coupling reaction | GO[ | Iodobenzene Benzene | Biaryl compounds | O-containing groups |
| GO[ | 1,3,5-Trimethoxy-benzene | Biaryl compounds | O-containing group, π-conjugated system and unpaired electrons | |
| Oxidative coupling of amines | Graphite oxide[ | Benzylamine | N-Benzylidene aniline | — |
| ba-GO[ | Benzylamine | N-Benzylidene aniline | The edge sites carboxyl | |
| PG[ | Benzylamine | N-Benzylidene aniline | Doped of P atoms | |
| BNHG[ | Benzylamine | N-Benzylidene aniline | Doped B and N atoms | |
| Friedel-Crafts alkylation | GO[ | Aromatics Styrene Alcohol | Diarylalkane | O-containing groups |
| GO[ | Aldehydes Indoles | Bis(indolyl)methanes | O-containing groups | |
| GO[ | Indole Ethers | 3,3'-Bisindolylmethane | — | |
| Friedel-Crafts acylation | Graphite[ | Aromatic compounds | Acylated products | — |
| Transesterification | GR-SO3H[ | Palm oil | Biodiesel | Benzenesulfonic acid groups |
| Esterification | GO-S[ | Oleic acid | Biodiesel | Sulfonic acid groups and carboxylic acid groups |
| Claisen-Schmidt Condensation | Graphite oxide[ | Phenylacetylene Benzyl alcohol | Chalcone | — |
| Michael reaction Knoevenagel reaction | GO-DETA[ | Benzaldehyde Malononitrile (E)-Chalcone | Benzylidenemalononitrile 2-(3-oxo-1,3-diphenylpropyl)malononitrile | Primary, secondary amino and carboxyl groups |
| Hydrolysis reaction | GO[ | Cellulose | Glucose | Hydroxyl and carboxyl groups |
| N-formylation reaction | GO[ | Tetrahydroisoquinoline | Formamide | Carboxylic acid group |
| Dehydrogenation reaction | GO[ | Tetrahydroisoquinoline | Dehydrogenation products | O-containing groups |
| Iodization reaction | GO[ | Benzothiazole | Iodination product | Unpaired electrons |
| Dehydration reaction | Graphite oxide[ | Benzyl alcohol | Poly(phenylene methylene) | O-containing groups |
| Graphite[ | Ethanol Silver nitrate | Silver cyanide | — |
| Reaction | Catalyst | Substrate | Product | Probable active site |
|---|---|---|---|---|
| Oxidation of alcohol | GO[ | Alcohol | Ketone/aldehyde | Epoxide groups |
| abGO[ | Benzylic alcohol | Benzaldehyde | Phenol hydroxyl groups | |
| NG-T[ | Benzylic alcohol | Benzaldehyde | Graphitic sp2-N sites | |
| BG[ | Benzylic alcohol | Benzaldehyde | B doping species (BC3) | |
| G1000[ | Benzylic alcohol | Benzaldehyde | Doped S and O atoms | |
| Oxidation of benzene | CCG[ | Benzene | Phenol | π-system of graphene and O-containing groups |
| Oxidation of cyclohexane | GO[ | Cyclohexane | Cyclohexanol Cyclohexanone Adipic acid | Carboxylic acid groups |
| Oxidation of alkyl- substituted arenes | NGO[ | Toluene | Benzoic acid | — |
| LC-N[ | Ethylbenzene | Acetophenone | C atoms adjacent to N atoms | |
| Oxidation of olefin | NG[ | Olefin | Alkylene oxide | Graphite N |
| Reduction of nitroaromatics | rGO[ | Nitrobenzene | Aniline | Edge sites |
| NG[ | Nitroaromatics | Aromatic amine | Graphitic N | |
| Reaction | Catalyst | Substrate | Product | Probable active site |
| NHG[ | Nitroaromatics | Aromatic amine | Doped N atoms | |
| NPG[ | Nitroaromatics | Aromatic amine | Doped N and P atoms | |
| Selective hydrogenation | Graphene[ | Acetylene Ethylene | Ethylene Ethane | Lewis acid-base pairs |
| Cross-coupling reaction | GO[ | Iodobenzene Benzene | Biaryl compounds | O-containing groups |
| GO[ | 1,3,5-Trimethoxy-benzene | Biaryl compounds | O-containing group, π-conjugated system and unpaired electrons | |
| Oxidative coupling of amines | Graphite oxide[ | Benzylamine | N-Benzylidene aniline | — |
| ba-GO[ | Benzylamine | N-Benzylidene aniline | The edge sites carboxyl | |
| PG[ | Benzylamine | N-Benzylidene aniline | Doped of P atoms | |
| BNHG[ | Benzylamine | N-Benzylidene aniline | Doped B and N atoms | |
| Friedel-Crafts alkylation | GO[ | Aromatics Styrene Alcohol | Diarylalkane | O-containing groups |
| GO[ | Aldehydes Indoles | Bis(indolyl)methanes | O-containing groups | |
| GO[ | Indole Ethers | 3,3'-Bisindolylmethane | — | |
| Friedel-Crafts acylation | Graphite[ | Aromatic compounds | Acylated products | — |
| Transesterification | GR-SO3H[ | Palm oil | Biodiesel | Benzenesulfonic acid groups |
| Esterification | GO-S[ | Oleic acid | Biodiesel | Sulfonic acid groups and carboxylic acid groups |
| Claisen-Schmidt Condensation | Graphite oxide[ | Phenylacetylene Benzyl alcohol | Chalcone | — |
| Michael reaction Knoevenagel reaction | GO-DETA[ | Benzaldehyde Malononitrile (E)-Chalcone | Benzylidenemalononitrile 2-(3-oxo-1,3-diphenylpropyl)malononitrile | Primary, secondary amino and carboxyl groups |
| Hydrolysis reaction | GO[ | Cellulose | Glucose | Hydroxyl and carboxyl groups |
| N-formylation reaction | GO[ | Tetrahydroisoquinoline | Formamide | Carboxylic acid group |
| Dehydrogenation reaction | GO[ | Tetrahydroisoquinoline | Dehydrogenation products | O-containing groups |
| Iodization reaction | GO[ | Benzothiazole | Iodination product | Unpaired electrons |
| Dehydration reaction | Graphite oxide[ | Benzyl alcohol | Poly(phenylene methylene) | O-containing groups |
| Graphite[ | Ethanol Silver nitrate | Silver cyanide | — |
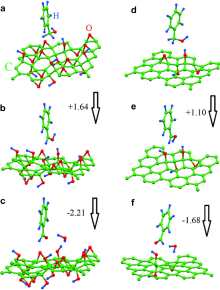


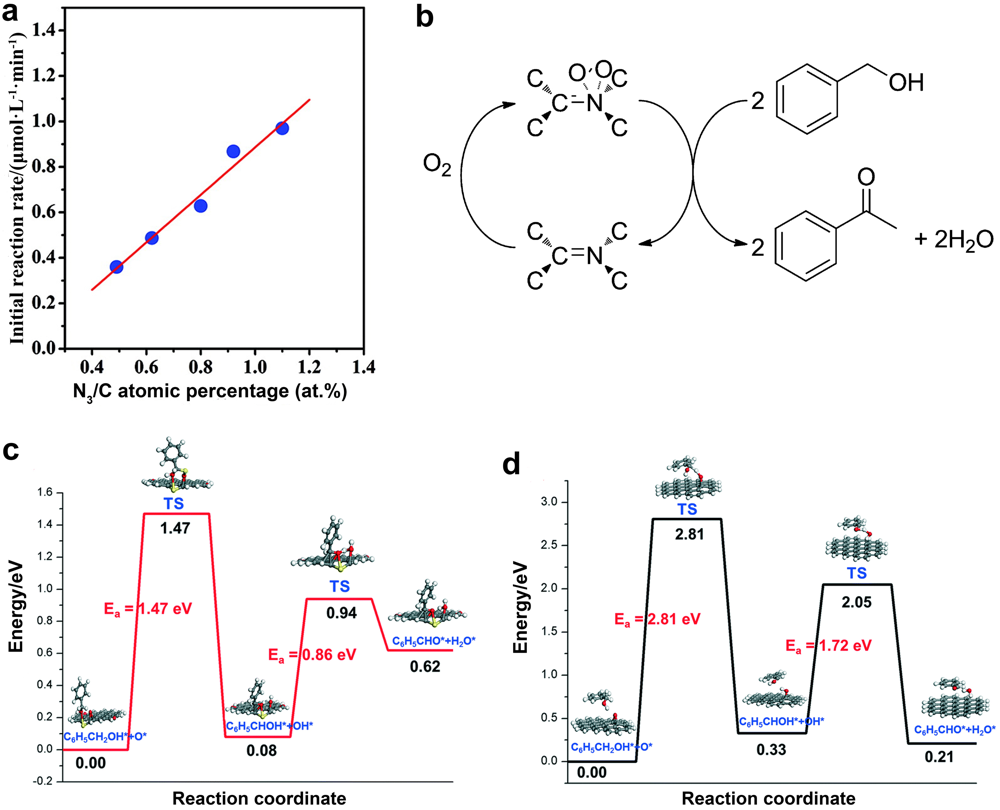
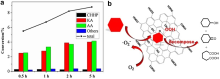


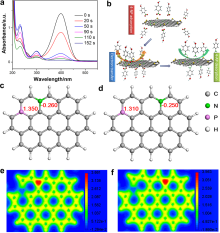

| [1] |
Chowdhury A. D.; Houben K.; Whiting G. T.; Chung S.-H.; Baldus M.; Weckhuysen B. M. Nat. Catal. 2018, 1, 23.
doi: 10.1038/s41929-017-0002-4 |
| [2] |
Bakandritsos A.; Kadam R. G.; Kumar P.; Zoppellaro G.; Medved M.; Tucek J.; Montini T.; Tomanec O.; Andryskova P.; Drahos B.; Varma R. S.; Otyepka M.; Gawande M. B.; Fornasiero P.; Zboril R. Adv. Mater. 2019, 31, 1900323.
doi: 10.1002/adma.v31.17 |
| [3] |
Zou Y. J.; Cheng H. J.; Wang H. N.; Huang R. X.; Xu Y. H.; Jiang J.; He Q.; Liu C. H.; Liu J. C.; Xiong J. M.; Yao J. N.; Huangfu X. L.; Ma J. Environ. Sci. Technol. 2020, 54, 7205.
doi: 10.1021/acs.est.0c00068 |
| [4] |
Pan L.; Xu M.-Y.; Feng L.-J.; Chen Q.; He Y.-J.; Han B.-H. Polym. Chem. 2016, 7, 2308.
doi: 10.1039/C6PY90040B |
| [5] |
Martin-Aranda R. M.; Cejka J. Top. Catal. 2010, 53, 141.
doi: 10.1007/s11244-009-9419-6 |
| [6] |
Nakagawa K. J. Jpn. Pet. Inst. 2019, 62, 53.
doi: 10.1627/jpi.62.53 |
| [7] |
Pentsak E. O.; Cherepanova V. A.; Ananikov V. P. ACS Appl. Mater. Interfaces 2017, 9, 36723.
doi: 10.1021/acsami.7b09173 |
| [8] |
Du Z. T.; Shao Z. H. Chem. Soc. Rev. 2013, 42, 1337.
doi: 10.1039/C2CS35258C |
| [9] |
Hu H. W.; Xin J. H.; Hu H.; Wang X. W.; Kong Y. Y. Appl. Catal. A-Gen. 2015, 492, 1.
doi: 10.1016/j.apcata.2014.11.041 |
| [10] |
Pandey R. K.; Prajapati V. K. Int. J. Biol. Macromol. 2018, 107, 1278.
doi: S0141-8130(17)33394-9 pmid: 29017884 |
| [11] |
Polshettiwar V.; Varma R. S. Green Chem. 2010, 12, 743.
doi: 10.1039/b921171c |
| [12] |
Su D. S.; Wen G. D.; Wu S. C.; Peng F.; Schlogl R. Angew. Chem. Int. Ed. 2017, 56, 936.
doi: 10.1002/anie.201600906 |
| [13] |
Su D. S.; Zhang J.; Frank B.; Thomas A.; Wang X. C.; Paraknowitsch J.; Schlogl R. ChemSusChem 2010, 3, 169.
doi: 10.1002/cssc.v3:2 |
| [14] |
Su D. S.; Perathoner S.; Centi G. Chem. Rev. 2013, 113, 5782.
doi: 10.1021/cr300367d |
| [15] |
Centi G.; Perathoner S.; Su D. S. Catal. Surv. Asia 2014, 18, 149.
doi: 10.1007/s10563-014-9172-0 |
| [16] |
Geim A. K.; Novoselov K. S. Nat. Mater. 2007, 6, 183.
pmid: 17330084 |
| [17] |
Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Science 2004, 306, 666.
pmid: 15499015 |
| [18] |
Chua C. K.; Pumera M. Chem. - Eur. J. 2015, 21, 12550.
doi: 10.1002/chem.201501383 |
| [19] |
Balandin A. A.; Ghosh S.; Bao W. Z.; Calizo I.; Teweldebrhan D.; Miao F.; Lau C. N. Nano Lett. 2008, 8, 902.
doi: 10.1021/nl0731872 |
| [20] |
Stoller M. D.; Park S. J.; Zhu Y. W.; An J. H.; Ruoff R. S. Nano Lett. 2008, 8, 3498.
doi: 10.1021/nl802558y pmid: 18788793 |
| [21] |
Chua C. K.; Pumera M. Chem. Soc. Rev. 2014, 43, 291.
doi: 10.1039/C3CS60303B |
| [22] |
Huang C. S.; Li Y. J.; Wang N.; Xue Y. R.; Zuo Z. C.; Liu H. B.; Li Y. L. Chem. Rev. 2018, 118, 7744.
doi: 10.1021/acs.chemrev.8b00288 |
| [23] |
Zuo Z. C.; Li Y. L. Joule 2019, 3, 899.
doi: 10.1016/j.joule.2019.01.016 |
| [24] |
Du Y. C.; Zhou W. D.; Gao J.; Pan X. Y.; Li Y. L. Acc. Chem. Res. 2020, 53, 459.
doi: 10.1021/acs.accounts.9b00558 |
| [25] |
Hui L.; Xue Y. R.; Yu H. D.; Liu Y. X.; Fang Y.; Xing C. Y.; Huang B. L.; Li Y. L. J. Am. Chem. Soc. 2019, 141, 10677.
doi: 10.1021/jacs.9b03004 |
| [26] |
Xue Y. R.; Huang B. L.; Yi Y. P.; Guo Y.; Zuo Z. C.; Li Y. J.; Jia Z. Y.; Liu H. B.; Li Y. L. Nat. Commun. 2018, 9, 1460.
doi: 10.1038/s41467-018-03896-4 |
| [27] |
Li L.; Zuo Z. C.; Wang F.; Gao J. C.; Cao A. M.; He F.; Li Y. L. Adv. Mater. 2020, 32, 2000140.
doi: 10.1002/adma.v32.14 |
| [28] |
Roy A. S.; Poulose A. C.; Bakandritsos A.; Varma R. S.; Otyepka M. Appl. Mater. Today 2021, 23, 101053.
|
| [29] |
Li X. T.; Wang J.; Duan X. G.; Li Y.; Fan X. B.; Zhang G. L.; Zhang F. B.; Peng W. C. ACS Catal. 2021, 11, 4848.
doi: 10.1021/acscatal.0c05089 |
| [30] |
Park M.; Lee J.; Kim B. S. Nanoscale 2021, 13, 10143.
doi: 10.1039/D1NR02025K |
| [31] |
Ahmad M. S.; Nishina Y. Nanoscale 2020, 12, 12210.
doi: 10.1039/D0NR02984J |
| [32] |
Wang Z. Y.; Pu Y.; Wang D.; Wang J. X.; Chen J. F. Front. Chem. Sci. Eng. 2018, 12, 855.
doi: 10.1007/s11705-018-1722-y |
| [33] |
Liu J. Q.; Tang J. G.; Gooding J. J. J. Mater. Chem. 2012, 22, 12435.
doi: 10.1039/c2jm31218b |
| [34] |
Daelemans B.; Bilbao N.; Dehaen W.; De Feyter S. Chem. Soc. Rev. 2021, 50, 2280.
doi: 10.1039/d0cs01294g pmid: 33404567 |
| [35] |
Srivastava S. K.; Pionteck J. J. Nanosci. Nanotechnol. 2015, 15, 1984.
pmid: 26413611 |
| [36] |
Dreyer D. R.; Todd A. D.; Bielawski C. W. Chem. Soc. Rev. 2014, 43, 5288.
doi: 10.1039/C4CS00060A |
| [37] |
Wang X. W.; Sun G. Z.; Routh P.; Kim D. H.; Huang W.; Chen P. Chem. Soc. Rev. 2014, 43, 7067.
doi: 10.1039/C4CS00141A |
| [38] |
Dimiev A. M.; Tour J. M. ACS Nano 2014, 8, 3060.
doi: 10.1021/nn500606a pmid: 24568241 |
| [39] |
Rosillo-Lopez M.; Lee T. J.; Bella M.; Hart M.; Salzmann C. G. RSC Adv. 2015, 5, 104198.
doi: 10.1039/C5RA23209K |
| [40] |
Wang C. I.; Periasamy A. P.; Chang H. T. Anal. Chem. 2013, 85, 3263.
doi: 10.1021/ac303613d |
| [41] |
Sun L. Chin. J. Chem. Eng. 2019, 27, 2251.
doi: 10.1016/j.cjche.2019.05.003 |
| [42] |
Dreyer D. R.; Park S.; Bielawski C. W.; Ruoff R. S. Chem. Soc. Rev. 2010, 39, 228.
doi: 10.1039/B917103G |
| [43] |
Lerf A.; He H.; Forster M. Phys. Chem. B 1998, 102, 4477.
doi: 10.1021/jp9731821 |
| [44] |
Yang J. H.; Yang D.; Tang P.; Ma D. Acta Phys.-Chim. Sin. 2016, 32, 75. (in Chinese)
doi: 10.3866/PKU.WHXB201512153 |
|
( 杨敬贺, 杨朵, 唐沛, 马丁, 物理化学学报, 2016, 32, 75.)
|
|
| [45] |
Loh K. P.; Bao Q. L.; Eda G.; Chhowalla M. Nat. Chem. 2010, 2, 1015.
doi: 10.1038/nchem.907 |
| [46] |
Wan W. B.; Li L. L.; Zhao Z. B.; Hu H.; Hao X. J.; Winkler D. A.; Xi L. C.; Hughes T. C.; Qiu J. S. Adv. Funct. Mater. 2014, 24, 4915.
doi: 10.1002/adfm.201303815 |
| [47] |
Eigler S.; Hu Y. C.; Ishii Y.; Hirsch A. Nanoscale 2013, 5, 12136.
doi: 10.1039/c3nr04332k |
| [48] |
Wang Y.; Li S. S.; Yang H. Y.; Luo J. RSC Adv. 2020, 10, 15328.
doi: 10.1039/D0RA01068E |
| [49] |
Su C. L.; Loh K. P. Acc. Chem. Res. 2013, 46, 2275.
doi: 10.1021/ar300118v |
| [50] |
Feng J. L.; Ye Y. Q.; Xiao M.; Wu G.; Ke Y. Chem. Pap. 2020, 74, 3767.
doi: 10.1007/s11696-020-01196-0 |
| [51] |
Larciprete R.; Fabris S.; Sun T.; Lacovig P.; Baraldi A.; Lizzit S. J. Am. Chem. Soc. 2011, 133, 17315.
doi: 10.1021/ja205168x pmid: 21846143 |
| [52] |
Xu C.; Yuan R. S.; Wang X. New Carbon Mater. 2014, 29, 61.
doi: 10.1016/S1872-5805(14)60126-8 |
| [53] |
Moon I. K.; Lee J.; Ruoff R. S.; Lee H. Nat. Commun. 2010, 1, 1.
|
| [54] |
Navalon S.; Dhakshinamoorthy A.; Alvaro M.; Garcia H. Chem. Rev. 2014, 114, 6179.
doi: 10.1021/cr4007347 pmid: 24867457 |
| [55] |
Yang J. H.; Sun G.; Gao Y. J.; Zhao H. B.; Tang P.; Tan J.; Lu A. H.; Ma D. Energy Environ. Sci. 2013, 6, 793.
doi: 10.1039/c3ee23623d |
| [56] |
Wang Z.; Pu Y.; Wang D.; Wang J.-X.; Chen J.-F. Front. Chem. Sci. Eng. 2018, 12, 855.
doi: 10.1007/s11705-018-1722-y |
| [57] |
Azlouk M.; Durmaz M.; Zor E.; Bingol H. Mater. Chem. Phys. 2020, 239, 122298.
doi: 10.1016/j.matchemphys.2019.122298 |
| [58] |
Liu H. T.; Liu Y. Q.; Zhu D. B. J. Mater. Chem. 2011, 21, 3335.
doi: 10.1039/C0JM02922J |
| [59] |
Zhang D. Y.; Lei L. Y.; Shang Y. H. Chem. Ind. Eng. Prog. 2016, 35, 831. (in Chinese)
|
|
( 张德懿, 雷龙艳, 尚永花, 化工进展, 2016, 35, 831.)
|
|
| [60] |
Zheng Y.; Jiao Y.; Jaroniec M.; Jin Y. G.; Qiao S. Z. Small 2012, 8, 3550.
doi: 10.1002/smll.201200861 pmid: 22893586 |
| [61] |
Feng L. Y.; Qin Z. Y.; Huang Y. J.; Peng K. S.; Wang F.; Yan Y. Y.; Chen Y. G. Sci. Total Environ. 2020, 698, 134239.
doi: 10.1016/j.scitotenv.2019.134239 |
| [62] |
Li X. H.; Antonietti M. Angew. Chem. Int. Ed. 2013, 52, 4572.
doi: 10.1002/anie.201209320 |
| [63] |
Patel M. A.; Luo F. X.; Khoshi M. R.; Rabie E.; Zhang Q.; Flach C. R.; Mendelsohn R.; Garfunkel E.; Szostak M.; He H. X. ACS Nano 2016, 10, 2305.
doi: 10.1021/acsnano.5b07054 |
| [64] |
Kong X.-K.; Chen C.-L.; Chen Q.-W. Chem. Soc. Rev. 2014, 43, 2841.
doi: 10.1039/C3CS60401B |
| [65] |
Guo X. L.; Qi W.; Liu W.; Yan P. Q.; Li F.; Liang C. H.; Su D. S. ACS Catal. 2017, 7, 1424.
doi: 10.1021/acscatal.6b02936 |
| [66] |
Dreyer D. R.; Jia H.-P.; Bielawski C. W. Angew. Chem., Int. Ed. 2010, 49, 6813.
|
| [67] |
Boukhvalov D. W.; Dreyer D. R.; Bielawski C. W.; Son Y. W. ChemCatChem 2012, 4, 1844.
doi: 10.1002/cctc.v4.11 |
| [68] |
Zhu S. H.; Cen Y. L.; Yang M. A.; Guo J.; Chen C. M.; Wang J. G.; Fan W. B. Appl. Catal., B-Environ. 2017, 211, 89.
doi: 10.1016/j.apcatb.2017.04.035 |
| [69] |
Long J. L.; Xie X. Q.; Xu J.; Gu Q.; Chen L. M.; Wang X. X. ACS Catal. 2012, 2, 622.
doi: 10.1021/cs3000396 |
| [70] |
Cheng W. J.; Liu X. T.; Li N.; Han J. T.; Li S. M.; Yu S. S. RSC Adv. 2018, 8, 11222.
doi: 10.1039/C8RA00290H |
| [71] |
Zhu S. H.; Chen Y. Y.; Gao X. Q.; Lv Z. X.; He Y.; Wang J. G.; Fan W. B. Catal. Sci. Technol. 2020, 10, 2786.
doi: 10.1039/C9CY02476J |
| [72] |
Xiao Y. P.; Liu J. C.; Xie K. H.; Wang W. B.; Fang Y. X. Mol. Catal. 2017, 431, 1.
|
| [73] |
Heidari M.; Sedrpoushan A.; Mohannazadeh F. Org. Process Res. Dev. 2017, 21, 641.
doi: 10.1021/acs.oprd.7b00056 |
| [74] |
Gao Y. J.; Hu G.; Zhong J.; Shi Z. J.; Zhu Y. S.; Su D. S.; Wang J. G.; Bao X. H.; Ma D. Angew. Chem. Int. Ed. 2013, 52, 2109.
doi: 10.1002/anie.v52.7 |
| [75] |
Li W. J.; Gao Y. J.; Chen W. L.; Tang P.; Li W. Z.; Shi Z. J.; Su D. S.; Wang J. G.; Ma D. ACS Catal. 2014, 4, 1261.
doi: 10.1021/cs500062s |
| [76] |
Gao Y. J.; Ma D.; Wang C. L.; Guan J.; Bao X. H. Chem. Commun. 2011, 47, 2432.
doi: 10.1039/C0CC04420B |
| [77] |
Yang F.; Chi C.; Wang C. X.; Wang Y.; Li Y. F. Green Chem. 2016, 18, 4254.
doi: 10.1039/C6GC00222F |
| [78] |
He Z. L.; Liu J.; Wang Q. J.; Zhao W.; Wen Z. P.; Chen J.; Manoj D.; Xie C. Y.; Xi J. B.; Yu J. X.; Tang C. Y.; Bai Z. W.; Wang S. J. Catal. 2019, 377, 199.
doi: 10.1016/j.jcat.2019.07.017 |
| [79] |
Xi J. B.; Wang Q. J.; Liu J.; Huan L.; He Z. L.; Qiu Y.; Zhang J.; Tang C. Y.; Xiao J.; Wang S. J. Catal. 2018, 359, 233.
doi: 10.1016/j.jcat.2018.01.003 |
| [80] |
Primo A.; Neatu F.; Florea M.; Parvulescu V.; Garcia H. Nat. Commun. 2014, 5, 5291.
doi: 10.1038/ncomms6291 pmid: 25342228 |
| [81] |
Gao Y. J.; Tang P.; Zhou H.; Zhang W.; Yang H. J.; Yan N.; Hu G.; Mei D. H.; Wang J. G.; Ma D. Angew. Chem., Int. Ed. 2016, 55, 3124.
doi: 10.1002/anie.201510081 |
| [82] |
Fang J. X.; Peng Z. Y.; Yang Y.; Wang J. W.; Guo J. Y.; Gong H. Asian J. Org. Chem. 2018, 7, 355.
doi: 10.1002/ajoc.v7.2 |
| [83] |
Huang H.; Huang J.; Liu Y. M.; He H. Y.; Cao Y.; Fan K. N. Green Chem. 2012, 14, 930.
doi: 10.1039/c2gc16681j |
| [84] |
Su C. L.; Acik M.; Takai K.; Lu J.; Hao S. J.; Zheng Y.; Wu P. P.; Bao Q. L.; Enoki T.; Chabal Y. J.; Loh K. P. Nat. Commun. 2012, 3, 1.
|
| [85] |
Yang F.; Fan X. X.; Wang C. X.; Yang W.; Hou L. Q.; Xu X. W.; Feng A. D.; Dong S.; Chen K.; Wang Y.; Li Y. F. Carbon 2017, 121, 443.
doi: 10.1016/j.carbon.2017.05.101 |
| [86] |
Hu F.; Patel M.; Luo F. X.; Flach C.; Mendelsohn R.; Garfunkel E.; He H. X.; Szostak M. J. Am. Chem. Soc. 2015, 137, 14473.
doi: 10.1021/jacs.5b09636 |
| [87] |
Wang Y. H.; Sang R.; Zheng Y.; Guo L.; Guan M.; Wu Y. Catal. Commun. 2017, 89, 138.
doi: 10.1016/j.catcom.2016.09.027 |
| [88] |
Peng X. J.; Zen Y.; Liu Q.; Liu L. X.; Wang H. S. Org. Chem. Front. 2019, 6, 3615.
doi: 10.1039/C9QO00926D |
| [89] |
Kodomari M.; Suzuki Y.; Yoshida K. Chem. Commun. 1997, 1567.
|
| [90] |
Nongbe M. C.; Ekou T.; Ekou L.; Yao K. B.; Le Grognec E.; Felpin F. X. Renew. Energ. 2017, 106, 135.
doi: 10.1016/j.renene.2017.01.024 |
| [91] |
Zhang H. L.; Luo X.; Shi K. Q.; Wu T.; He F.; Zhou S. B.; Chen G. Z.; Peng C. ChemSusChem 2017, 10, 3352.
doi: 10.1002/cssc.201700950 |
| [92] |
Jia H. P.; Dreyer D. R.; Bielawski C. W. Adv. Synth. Catal. 2011, 353, 528.
doi: 10.1002/adsc.201000748 |
| [93] |
Yang A. W.; Li J. J.; Zhang C.; Zhang W. Q.; Ma N. Appl. Surf. Sci. 2015, 346, 443.
doi: 10.1016/j.apsusc.2015.04.033 |
| [94] |
Zhao X. C.; Wang J.; Chen C. M.; Huang Y. Q.; Wang A. Q.; Zhang T. Chem. Commun. 2014, 50, 3439.
doi: 10.1039/c3cc49634a |
| [95] |
Ma J.; Zhang J. Y.; Zhou X.; Wang J. W.; Gong H. J. Iran. Chem. Soc. 2018, 15, 2851.
doi: 10.1007/s13738-018-1471-3 |
| [96] |
Zhang J. Y.; Chen S. Y.; Chen F. F.; Xu W. S.; Deng G. J.; Gong H. Adv. Synth. Catal. 2017, 359, 2358.
doi: 10.1002/adsc.v359.14 |
| [97] |
Zhang J. Y.; Li S. G.; Deng G. J.; Gong H. ChemCatChem 2018, 10, 376.
doi: 10.1002/cctc.v10.2 |
| [98] |
Dreyer D. R.; Jarvis K. A.; Ferreira P. J.; Bielawski C. W. Macromolecules 2011, 44, 7659.
doi: 10.1021/ma201306x |
| [99] |
Xiao D.; Wang W. C.; Gai Y. Z.; Zhao Y. Sci. Rep. 2018, 8, 1750.
doi: 10.1038/s41598-018-20238-y |
| [100] |
Tang P.; Hu G.; Li M. Z.; Ma D. ACS Catal. 2016, 6, 6948.
doi: 10.1021/acscatal.6b01668 |
| [101] |
Wen G. D.; Gu Q. Q.; Liu Y. F.; Schlogl R.; Wang C. X.; Tian Z. J.; Su D. S. Angew. Chem. Int. Ed. 2018, 57, 16898.
doi: 10.1002/anie.v57.51 |
| [102] |
Chen B.; Wang L. Y.; Gao S. ACS Catal. 2015, 5, 5851.
doi: 10.1021/acscatal.5b01479 |
| [103] |
Mukherjee A.; Nerush A.; Leitus G.; Shimon L. J. W.; Ben David Y.; Jalapa N. A. E.; Milstein D. J. Am. Chem. Soc. 2016, 138, 4298.
doi: 10.1021/jacs.5b13519 pmid: 26924231 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||