Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (1): 16-25.DOI: 10.6023/A23080375 Previous Articles Next Articles
Article
投稿日期:2023-08-10
发布日期:2023-10-23
基金资助:
Xiangguo Teng, Liangwei Zhang, Xiaoyu Han, Guowei Li, Jicui Dai( )
)
Received:2023-08-10
Published:2023-10-23
Contact:
E-mail: Supported by:Share
Xiangguo Teng, Liangwei Zhang, Xiaoyu Han, Guowei Li, Jicui Dai. Study on the Polyamine Thin Film Composite Membrane for Vanadium Battery[J]. Acta Chimica Sinica, 2024, 82(1): 16-25.


| 膜 | 质量损失率/% | 浸泡前钒离子 渗透系数/ (×10-7 cm2•min-1) | 浸泡后钒离子 渗透系数/ (×10-7 cm2•min-1) | 钒离子渗透系数 损失率/% |
|---|---|---|---|---|
| M0 | 2.85 | 30.89 | 33.65 | 8.93 |
| MT | 1.66 | 3.17 | 3.31 | 4.42 |
| N 115 | 1.73 | 10.91 | 11.44 | 4.86 |
| 膜 | 质量损失率/% | 浸泡前钒离子 渗透系数/ (×10-7 cm2•min-1) | 浸泡后钒离子 渗透系数/ (×10-7 cm2•min-1) | 钒离子渗透系数 损失率/% |
|---|---|---|---|---|
| M0 | 2.85 | 30.89 | 33.65 | 8.93 |
| MT | 1.66 | 3.17 | 3.31 | 4.42 |
| N 115 | 1.73 | 10.91 | 11.44 | 4.86 |
| 膜 | 电流密度/ (mA•cm-2) | CE/% | VE/% | EE/% | 文献 |
|---|---|---|---|---|---|
| 全氟磺酸填充多孔聚乙烯 | 60 | ≈93 | ≈85 | ≈79 | [ |
| 三维聚偏氟乙烯多孔膜 | 100 | 96 | 85 | 82 | [ |
| 聚苯并咪唑/聚乙烯吡咯烷酮 | 100 | 99.26 | 74.15 | 74.79 | [ |
| 磺化聚酰亚胺/聚乙烯吡咯烷酮 | 20 | 95 | 71 | 67.5 | [ |
| Nafion填充多孔聚偏氟乙烯膜 | 80 | ≈93 | ≈73 | ≈68 | [ |
| 二维MFI 分子筛膜 | 80 | 97.5 | 77.2 | 75.2 | [ |
| 聚酰胺薄层 复合膜 | 80 | 99.2 | 92.8 | 92.1 | [ |
| 多巴胺改性的聚酰胺薄层复合膜 | 80 | 95.7 | 86.8 | 83.1 | [ |
| 层层自组装改性聚醚砜多孔膜 | 80 | 98 | 89.8 | 88 | [ |
| 聚胺薄层复合膜 | 80 | 97.9 | 79.8 | 78.1 | 本文 |
| 膜 | 电流密度/ (mA•cm-2) | CE/% | VE/% | EE/% | 文献 |
|---|---|---|---|---|---|
| 全氟磺酸填充多孔聚乙烯 | 60 | ≈93 | ≈85 | ≈79 | [ |
| 三维聚偏氟乙烯多孔膜 | 100 | 96 | 85 | 82 | [ |
| 聚苯并咪唑/聚乙烯吡咯烷酮 | 100 | 99.26 | 74.15 | 74.79 | [ |
| 磺化聚酰亚胺/聚乙烯吡咯烷酮 | 20 | 95 | 71 | 67.5 | [ |
| Nafion填充多孔聚偏氟乙烯膜 | 80 | ≈93 | ≈73 | ≈68 | [ |
| 二维MFI 分子筛膜 | 80 | 97.5 | 77.2 | 75.2 | [ |
| 聚酰胺薄层 复合膜 | 80 | 99.2 | 92.8 | 92.1 | [ |
| 多巴胺改性的聚酰胺薄层复合膜 | 80 | 95.7 | 86.8 | 83.1 | [ |
| 层层自组装改性聚醚砜多孔膜 | 80 | 98 | 89.8 | 88 | [ |
| 聚胺薄层复合膜 | 80 | 97.9 | 79.8 | 78.1 | 本文 |
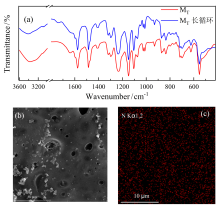

| [1] |
Cunha, Á.; Martins, J.; Rodrigues, N.; Brito, F. P. Int. J. Energ. Res. 2015, 39, 889.
doi: 10.1002/er.v39.7 |
| [2] |
Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. J. Phys. Chem. Lett. 2013, 4, 1281.
doi: 10.1021/jz4001032 |
| [3] |
Yang, Z.; Zhang, J.; Kintner-Meyer, M. C. W.; Lu, X.; Choi, D.; Lemmon, J. P.; Liu, J. Chem. Rev. 2011, 111, 3577.
doi: 10.1021/cr100290v |
| [4] |
Soloveichik, G. L. Chem. Rev. 2015, 115, 11533.
doi: 10.1021/cr500720t pmid: 26389560 |
| [5] |
Kear, G.; Shah, A. A.; Walsh, F. C. Int. J. Energ. Res. 2012, 36, 1105.
doi: 10.1002/er.v36.11 |
| [6] |
Zhang, H.-M.; Wang, X.-L.; 2013, 2, 281. (in Chinese)
|
|
(张华民, 王晓丽, 储能科学与技术, 2013, 2, 281.)
doi: 10.3969/j.issn.2095-4239.2013.03.014 |
|
| [7] |
Skyllas-Kazacos, M.; Rychcik, M.; Robins, R. G.; Fane, A. G.; Green, M. A. J. Electrochem. Soc. 1986, 133, 1057.
doi: 10.1149/1.2108706 |
| [8] |
Rychcik, M.; Skyllas-Kazacos, M. J. Power Sources 1988, 22, 59.
doi: 10.1016/0378-7753(88)80005-3 |
| [9] |
Jia, Z.-J.; Song, S.-Q.; Wang, B.-G. 2012, 1, 50. (in Chinese)
|
|
(贾志军, 宋士强, 王保国, 储能科学与技术, 2012, 1, 50.)
|
|
| [10] |
Liu, M.-Y.; Han, L.-W.; Zheng, J.-T.; Xu, H.-W.; Cao, C.-Z. Chinese Journal of Power Sources 2016, 40, 1330. (in Chinese)
|
|
(刘明义, 韩临武, 郑建涛, 徐海卫, 曹传钊, 电源技术, 2016, 40, 1330.)
|
|
| [11] |
Doan, T. N. L.; Hoang, T. K. A.; Chen, P. RSC Adv 2015, 5, 72805.
doi: 10.1039/C5RA05914C |
| [12] |
Zhang, B.-G.; Zhang, S.-H.; Xing, D.-B.; Yin, C.-X.; Han, R.-L.; Jian, X.-G. 2011, 69, 583. (in Chinese)
|
|
(张本贵, 张守海, 邢东博, 尹春香, 韩润林, 蹇锡高, 化学学报, 2011, 69, 583.)
|
|
| [13] |
Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T. M.; Skyllas-Kazacos, M. Membranes-Basel 2012, 2, 275.
|
| [14] |
Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T. M.; Ukil, A.; Zhao, J. Appl. Energ. 2019, 238, 202.
doi: 10.1016/j.apenergy.2018.12.087 |
| [15] |
Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. ChemSusChem 2011, 4, 1388.
pmid: 22102992 |
| [16] |
Ding, Y.; Wang, L.-H.; Han, X.-T.; 2013, 1476. (in Chinese)
|
|
(丁跃, 王丽华, 韩旭彤, 高分子学报, 2013, 1476.)
|
|
| [17] |
Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Energ. Environ. Sci. 2011, 4, 1147.
doi: 10.1039/c0ee00770f |
| [18] |
Fetyan, A.; Benetho, B. P.; Bamgbopa, M. O. J. Energy Chem. 2023, 81, 64.
doi: 10.1016/j.jechem.2023.01.058 |
| [19] |
Wang, F.-R.; Jiang, F.-J. Acta Chim. Sinica 2021, 79, 1123. (in Chinese)
doi: 10.6023/A21050231 |
|
(王斐然, 蒋峰景, 化学学报, 2021, 79, 1123.)
doi: 10.6023/A21050231 |
|
| [20] |
Ahn, Y.; Kim, D. Energy Storage Mater. 2020, 31, 105.
|
| [21] |
Wang, F.-R.; Jiang, F.-J. Prog. Chem. 2021, 33, 462. (in Chinese)
|
|
(王斐然, 蒋峰景, 化学进展, 2021, 33, 462.)
doi: 10.7536/PC200567 |
|
| [22] |
Zhang, S.; Liu, Q.-H.; John, L.; Miao, P.; Jiang, Z. Y.; 2020, 40, 50. (in Chinese)
|
|
(张赛, 刘庆华, John, L. 缪平, 姜忠义, 现代化工, 2020, 40, 50.)
|
|
| [23] |
Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Zhang, J. Energ. Environ. Sci. 2011, 4, 1676.
doi: 10.1039/c1ee01117k |
| [24] |
Zhou, X.; Xue, R.; Zhong, Y.; Zhang, Y.; Jiang, F. J. Membrane Sci. 2020, 595, 117614.
doi: 10.1016/j.memsci.2019.117614 |
| [25] |
Lu, W.; Yuan, Z.; Zhao, Y.; Qiao, L.; Zhang, H.; Li, X. Energy Storage Mater. 2018, 10, 40.
|
| [26] |
Mögelin, H.; Yao, G.; Zhong, H.; Dos Santos, A. R.; Barascu, A.; Meyer, R.; Krenkel, S.; Wassersleben, S.; Hickmann, T.; Enke, D.; Turek, T.; Kunz, U. J. Power Sources 2018, 377, 18.
doi: 10.1016/j.jpowsour.2017.12.001 |
| [27] |
Zhao, Y.; Yuan, Z.; Lu, W.; Li, X.; Zhang, H. J. Power Sources 2017, 342, 327.
doi: 10.1016/j.jpowsour.2016.12.058 |
| [28] |
Chae, I. S.; Luo, T.; Moon, G. H.; Ogieglo, W.; Kang, Y. S.; Wessling, M. Adv. Energy Mater. 2016, 6, 1600517.
doi: 10.1002/aenm.v6.16 |
| [29] |
Lu, W.; Yuan, Z.; Zhao, Y.; Zhang, H.; Zhang, H.; Li, X. Chem. Soc. Rev. 2017, 46, 2199.
doi: 10.1039/C6CS00823B |
| [30] |
Wu, J.; Dai, Q.; Zhang, H.; Li, X. ChemSusChem 2020, 13, 3805.
doi: 10.1002/cssc.v13.15 |
| [31] |
Xu, Z.; Huang, K. Chem. Ind. Eng. Prog. 2022, 41, 1569. (in Chinese)
|
|
(徐至, 黄康, 化工进展, 2022, 41, 1569.)
doi: 10.16085/j.issn.1000-6613.2021-2213 |
|
| [32] |
Zhang, H.-J.; Shen, J.-N.; Gao, C.-J.; 2017, 27, 6. (in Chinese)
|
|
(张慧娟, 沈江南, 高从堦, 过滤与分离, 2017, 27, 6.)
|
|
| [33] |
Xu, G.; Wang, J.; Li, C. Desalination 2013, 328, 83.
doi: 10.1016/j.desal.2013.08.022 |
| [34] |
Dai, Q.; Liu, Z.; Huang, L.; Wang, C.; Zhao, Y.; Fu, Q.; Zheng, A.; Zhang, H.; Li, X. Nat. Commun. 2020, 11, 13.
doi: 10.1038/s41467-019-13704-2 |
| [35] |
Liang, S.; Xu, G.; Jin, Y.; Wu, Z.; Cai, Z.; Zhao, N.; Wu, Z. J. Membrane Sci. 2015, 476, 475.
doi: 10.1016/j.memsci.2014.12.003 |
| [36] |
Yao, C. W.; Burford, R. P.; Fane, A. G.; Fell, C. J. D.; Mcdonogh, R. M. J. Appl. Polym. Sci. 1987, 34, 2399.
doi: 10.1002/app.1987.070340706 |
| [37] |
Dalwani, M.; Benes, N. E.; Bargeman, G.; Stamatialis, D.; Wessling, M. J. Membrane Sci. 2011, 372, 228.
doi: 10.1016/j.memsci.2011.02.012 |
| [38] |
Lee, K. P.; Zheng, J.; Bargeman, G.; Kemperman, A. J. B.; Benes, N. E. J. Membrane Sci. 2015, 478, 75.
doi: 10.1016/j.memsci.2014.12.045 |
| [39] |
Ma, X.; Wen, X.; Gu, S.; Xu, Z.; Zhang, J. J. Membrane Sci. 2013, 430, 62.
doi: 10.1016/j.memsci.2012.11.073 |
| [40] |
Lu, W.; Yuan, Z.; Li, M.; Li, X.; Zhang, H.; Vankelecom, I. Adv. Funct. Mater. 2017, 27, 1604587.
doi: 10.1002/adfm.v27.4 |
| [41] |
Lau, W.; Ismail, A. F. Desalination 2009, 249, 996.
doi: 10.1016/j.desal.2009.09.016 |
| [42] |
Jaafar, J.; Ismail, A. F.; Mustafa, A. Mat. Sci. Eng. A.-Struct. 2007, 460, 475.
|
| [43] |
Daud, S. N. S. S.; Norddin, M. N. A. M.; Jaafar, J.; Sudirman, R.; Othman, M. H. D.; Ismail, A. F. Arab. J. Sci. Eng. 2021, 46, 6189.
doi: 10.1007/s13369-020-04898-5 |
| [44] |
Lau, W. J.; Ismail, A. F. J. Membrane Sci. 2009, 334, 30.
doi: 10.1016/j.memsci.2009.02.012 |
| [45] |
Bai, L.; Wang, M.; Li, Z.; Yang, H.; Peng, Z.; Zhao, Y. J. Membrane Sci. 2022, 643, 120012.
doi: 10.1016/j.memsci.2021.120012 |
| [46] |
Jiang, Z.; Miao, J.; He, Y.; Hong, X.; Tu, K.; Wang, X.; Chen, S.; Yang, H.; Zhang, L.; Zhang, R. RSC Adv. 2019, 9, 37546.
doi: 10.1039/C9RA06528H |
| [47] |
Zhu, X.; Cheng, X.; Luo, X.; Liu, Y.; Xu, D.; Tang, X.; Gan, Z.; Yang, L.; Li, G.; Liang, H. Environ. Sci. Technol. 2020, 54, 6365.
doi: 10.1021/acs.est.9b06779 |
| [48] |
Teng, X.; Guo, Y.; Liu, D.; Li, G.; Yu, C.; Dai, J. J. Membrane Sci. 2020, 601, 117906.
doi: 10.1016/j.memsci.2020.117906 |
| [49] |
Zhang, B.; Liu, S.; Wang, L. H.; Han, X. T.; 2015, 418. (in Chinese)
|
|
(张斌, 刘帅, 王丽华, 韩旭彤, 高分子学报, 2015, 418.)
|
|
| [50] |
Tian, J.; Chang, H.; Gao, S.; Zhang, R. Desalination 2020, 491, 114499.
doi: 10.1016/j.desal.2020.114499 |
| [51] |
Thong, P. T.; Ajeya, K. V.; Dhanabalan, K.; Roh, S.; Son, W.; Park, S.; Jung, H. J. Power Sources 2022, 521, 230912.
doi: 10.1016/j.jpowsour.2021.230912 |
| [52] |
Song, X.-P.; Liu, J.-Y.; Wang, L.-H.; Han, X.-T.; Huang, Q.-L. 2019, 40, 1543. (in Chinese)
|
|
(宋西鹏, 刘金宇, 王丽华, 韩旭彤, 黄庆林, 高等学校化学学报, 2019, 40, 1543.)
doi: 10.7503/cjcu20180720 |
|
| [53] |
Dalal, U.; Kapoor, M.; Verma, A. Energ. Fuel. 2023, 37, 13457.
doi: 10.1021/acs.energyfuels.3c01932 |
| [54] |
Zhang, D.; Huang, K.; Xia, Y.; Cao, H.; Dai, L.; Qu, K.; Xiao, L.; Fan, Y.; Xu, Z. Angew. Chem. Int. Ed. 2023, 62, e2023109.
|
| [55] |
Guo, C.-S. Ph.D. Dissertation, Tiangong University, Tianjin, 2022. (in Chinese)
|
|
(郭昌盛, 博士论文, 天津工业大学,天津, 2022.)
|
|
| [56] |
Shi, Q.; Ni, L.; Zhang, Y.; Feng, X.; Chang, Q.; Meng, J. J. Mater. Chem. A 2017, 5, 13610.
doi: 10.1039/C7TA02552A |
| [57] |
Hu, P.; He, J.; Tian, B.; Xu, Z.; Yuan, T.; Sun, H.; Li, P.; Niu, Q. J. Desalination 2020, 496, 114340.
doi: 10.1016/j.desal.2020.114340 |
| [58] |
Zhu, X.; Tang, X.; Luo, X.; Yang, Z.; Cheng, X.; Gan, Z.; Xu, D.; Li, G.; Liang, H. J. Membrane Sci. 2021, 618, 118738.
doi: 10.1016/j.memsci.2020.118738 |
| [59] |
Zeng, H.; Li, Y.; Wang, C. Sep. Purif. Technol. 2020, 236, 116258.
doi: 10.1016/j.seppur.2019.116258 |
| [60] |
Aravind, K.; Sangeetha, D. Int. J. Polym. Mater. Polym. Biomat. 2014, 64, 220.
doi: 10.1080/00914037.2014.936594 |
| [61] |
Zhang, R.; Yu, S.; Shi, W.; Zhu, J.; Van der Bruggen, B. Sep. Purif. Technol. 2019, 215, 670.
doi: 10.1016/j.seppur.2019.01.045 |
| [62] |
Li, G.-W. M.S. Thesis, Harbin Institute of Technology, Haerbin, 2019. (in Chinese)
|
|
(李郭威, 硕士论文, 哈尔滨工业大学,哈尔滨, 2019.)
|
|
| [63] |
Hasbullah, N.; Sekak, K. A.; Ibrahim, I.; Mahmood, M. R.; Soga, T.; Nagaoka, S.; Mamat, M. H.; Jafar, S. M. Aip Conference Proceedings 2016, 1733, 020049.
|
| [64] |
Dong, Y.-F.; Tan, X.-L.; Guo, Q.; Li, X.; Li, D.; Li, M. Physical Testing and Chemical Analysis Part A: Physical Testing 2011, 47, 535 (in Chinese)
|
|
(董云凤, 谭孝林, 郭强, 李夏, 李丹, 李萌, 理化检验(物理分册), 2011, 47, 535.)
|
|
| [65] |
Yuan, Z.; Zhu, X.; Li, M.; Lu, W.; Li, X.; Zhang, H. Angew. Chem. Int. Ed. 2016, 55, 3058.
doi: 10.1002/anie.v55.9 |
| [66] |
Zheng, L.; Wang, H.; Niu, R.; Zhang, Y.; Shi, H. Electrochim. Acta 2018, 282, 437.
doi: 10.1016/j.electacta.2018.06.083 |
| [67] |
Wang, N.-F. Ph.D. Dissertation, Central South University, Changsha, 2012. (in Chinese)
|
|
(汪南方, 博士论文, 中南大学, 长沙, 2012.)
|
|
| [68] |
Wang, F.; Zhang, Z.; Jiang, F. J. Power Sources 2021, 506, 230234.
doi: 10.1016/j.jpowsour.2021.230234 |
| [1] | Li Yang, Qi Limin. Advances in Fabrication of Two-dimensionally Ordered Porous Membranes by Nanosphere Lithography at the Gas-liquid Interface [J]. Acta Chim. Sinica, 2015, 73(9): 869-876. |
| [2] | Huang Xianwei, Deng Jiyong, Xu L?, Shen Ping, Zhao Bin, Tan Songting. Preparation of Polymer/TiO2 Hybrid Nanofibers Microporous Membranes and Its Application in Dye-Sensitized Solar Cells [J]. Acta Chimica Sinica, 2012, 70(15): 1604-1610. |
| [3] | FENG Xiao-Ming, HUANG Xian-Wei, HUANG Hui, SHEN Ping, ZHAO Bin, TAN Song-Ting. Study of the TiO2 Nanofibers Network Microporous Film for Organic Dye-sensitized Solar Cells [J]. Acta Chimica Sinica, 2010, 68(11): 1123-1129. |
| [4] | JU Min, LIU Shu-Wen, ZHOU Chun-Qiong. Synthesis and Studies on DNA-binding of a New Nickel Complex with Pyrrole and Polyamine [J]. Acta Chimica Sinica, 2010, 68(06): 481-486. |
| [5] | WANG Fei WEI Qi* WANG Yan-Li YU Chun-Xiao ZHONG Zhen-Xing LI Qun-Yan NIE Zuo-Ren. Preparation and Characterization of Hydrophobic Microporous Silica Membranes Modified by Fluorocarbon Groups [J]. Acta Chimica Sinica, 2008, 66(1): 44-48. |
| [6] | XIAO Wen-Jun; LI Zhao-Hui; HUANG Zai-Bo; TAN Song-Ting*,1,2. Preparation of the TiO2/P(VdF-HFP) Hybrid Fiber Microporous Film and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2007, 65(19): 2097-2102. |
| [7] | SU Bi-Tao*; TONG Yong-Chun; WANG Ke; BAI Jie; DONG Na; MU Hong-Mei; MIN Shi-Xiong; SHE Shi-Xiong; LEI Zi-Qiang. Preparation of Polyfurfural Nanospheres by Interfacial Polymerization [J]. Acta Chimica Sinica, 2007, 65(12): 1161-1164. |
| [8] | YANG Qian-Rong, CHEN Xin*, SHAO Zheng-Zhong. Preparation of Natural Polyelectrolytical Chitosan/Carboxymethyl Chitosan Complex Membrane [J]. Acta Chimica Sinica, 2005, 63(4): 259-262. |
| [9] | DAI Li-Ping, TAO Zhu, ZHU Qian-Jiang, XUE Sai-Feng, ZHANG Jian-Xin, ZHOU Xin. Investigation of Self-assembled Inclusion Complexes of Cucurbit[n=5~8]urils with Polyamines Guests [J]. Acta Chimica Sinica, 2004, 62(24): 2431-2440. |
| [10] | Wang Zhongming;Zhou Zhifen;Lin Huakuan;Xu Meng;Liu Tianfu;Zhu Shourong;Sun Hongwei;Chen Rongti. Studies on the stability of rare earth ion -N,N'-1,10- phenanthroline -2,9-dimethanamine binary complexes [J]. Acta Chimica Sinica, 2001, 59(5): 701-706. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||