Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (1): 1-4.DOI: 10.6023/A23100472 Previous Articles Next Articles
Communication
投稿日期:2023-10-27
发布日期:2023-12-20
基金资助:
Weiyang Gao, Weichao Deng, Yang Gao, Renxiao Liang, Yixia Jia( )
)
Received:2023-10-27
Published:2023-12-20
Contact:
E-mail: Supported by:Share
Weiyang Gao, Weichao Deng, Yang Gao, Renxiao Liang, Yixia Jia. Intermolecular Enantioselective Dearomative Oxidative Heck Reaction of Indoles[J]. Acta Chimica Sinica, 2024, 82(1): 1-4.
| Entry | Ligand | Co-oxidant | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|
| 1 | 1,10-Phen | — | DMF | 13 | — |
| 2 | 1,10-Phen | — | THF | n.d. | — |
| 3 | 1,10-Phen | — | MeCN | n.d. | — |
| 4 | 1,10-Phen | — | MeOH | n.d. | — |
| 5 | 1,10-Phen | — | NMP | 21 | — |
| 6 | 1,10-Phen | — | 1,4-Dioxane | 6 | — |
| 7 | 1,10-Phen | FeCl3 | NMP | n.d. | — |
| 8 | 1,10-Phen | CuCl2 | NMP | 15 | — |
| 9 | 1,10-Phen | BQ | NMP | 64 | — |
| 10 | 1,10-Phen | DDQ | NMP | 47 | — |
| 11 | 1,10-Phen | 2,6-DMBQ | NMP | 56 | — |
| 12 | L1 | BQ | NMP | 51 | rac |
| 13 | L2 | BQ | NMP | 55 | 19 |
| 14 | L3 | BQ | NMP | 32 | 3 |
| 15 | L4 | BQ | NMP | 60 | 13 |
| 16 | L5 | BQ | NMP | 72 | 17 |
| 17 | L6 | BQ | NMP | 27 | 44 |
| 18 | L7 | BQ | NMP | 64 | 53 |
| 19 | L8 | BQ | NMP | 60 | 70 |
| 20 | L9 | BQ | NMP | 73 | 75 |
| 21 | L10 | BQ | NMP | 49 | 20 |
| 22d | L9 | BQ | NMP | 51 | 73 |
| Entry | Ligand | Co-oxidant | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|
| 1 | 1,10-Phen | — | DMF | 13 | — |
| 2 | 1,10-Phen | — | THF | n.d. | — |
| 3 | 1,10-Phen | — | MeCN | n.d. | — |
| 4 | 1,10-Phen | — | MeOH | n.d. | — |
| 5 | 1,10-Phen | — | NMP | 21 | — |
| 6 | 1,10-Phen | — | 1,4-Dioxane | 6 | — |
| 7 | 1,10-Phen | FeCl3 | NMP | n.d. | — |
| 8 | 1,10-Phen | CuCl2 | NMP | 15 | — |
| 9 | 1,10-Phen | BQ | NMP | 64 | — |
| 10 | 1,10-Phen | DDQ | NMP | 47 | — |
| 11 | 1,10-Phen | 2,6-DMBQ | NMP | 56 | — |
| 12 | L1 | BQ | NMP | 51 | rac |
| 13 | L2 | BQ | NMP | 55 | 19 |
| 14 | L3 | BQ | NMP | 32 | 3 |
| 15 | L4 | BQ | NMP | 60 | 13 |
| 16 | L5 | BQ | NMP | 72 | 17 |
| 17 | L6 | BQ | NMP | 27 | 44 |
| 18 | L7 | BQ | NMP | 64 | 53 |
| 19 | L8 | BQ | NMP | 60 | 70 |
| 20 | L9 | BQ | NMP | 73 | 75 |
| 21 | L10 | BQ | NMP | 49 | 20 |
| 22d | L9 | BQ | NMP | 51 | 73 |
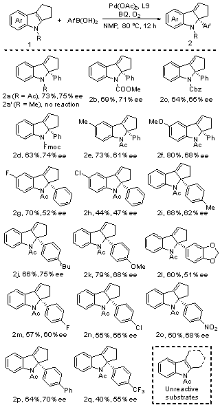
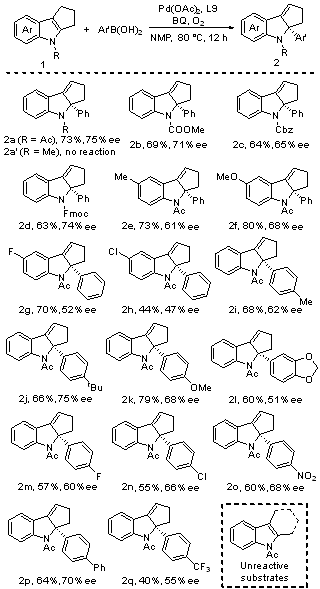
| [1] |
(a) Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581.
doi: 10.1246/bcsj.44.581 |
|
(b) Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320.
doi: 10.1021/jo00979a024 |
|
| [2] |
For selected reviews of Heck reaction, see: (a) Heck, R. F. Acc. Chem. Res. 1979, 12, 146.
doi: 10.1021/ar50136a006 |
|
(b) Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2.
doi: 10.1021/ar00049a001 |
|
|
(c) Le Bras, J.; Muzart, J. Chem. Rev. 2011, 111, 1170.
doi: 10.1021/cr100209d |
|
|
(d) Paul, D.; Das, S.; Saha, S.; Sharma, H.; Goswami, R. K. Eur. J. Org. Chem. 2021, 2021, 2057.
doi: 10.1002/ejoc.v2021.14 |
|
| [3] |
(a) Sato, Y.; Sodeoka, M.; Shibasaki, M. J. Org. Chem. 1989, 54, 4738.
doi: 10.1021/jo00281a007 |
|
(b) Carpenter, N. E.; Kucera, D. J.; Overman, L. E. J. Org. Chem. 1989, 54, 5846.
doi: 10.1021/jo00286a009 |
|
| [4] |
For selected reviews of asymmetric Heck reaction, see: (a) Dounay, A. B.; Overman, L. E.; Chem. Rev. 2003, 103, 2945.
pmid: 21677934 |
|
(b) Shibasaki, M.; Vogl, E. M.; Ohshima, T. Adv. Synth. Catal. 2004, 346, 1533.
doi: 10.1002/adsc.v346:13/15 pmid: 21677934 |
|
|
(c) Cartney, D. M.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122.
doi: 10.1039/c1cs15101k pmid: 21677934 |
|
|
(d) Li, H.; Ding, C.; Xu, B.; Hou, X. Acta Chim. Sinica 2014, 72, 765. (in Chinese)
doi: 10.6023/A14040329 pmid: 21677934 |
|
|
(李浩, 丁昌华, 许斌, 侯雪龙, 化学学报, 2014, 72, 765.)
doi: 10.6023/A14040329 pmid: 21677934 |
|
|
(e) Xie, J.-Q.; Liang, R.-X.; Jia, Y.-X. Chin. J. Chem. 2021, 39, 710.
doi: 10.1002/cjoc.v39.3 pmid: 21677934 |
|
|
(f) Li, S.; Chen, Q.; Zhang, Z.-M.; Zhang, J. Green Synth. Catal. 2021, 2, 374.
pmid: 21677934 |
|
| [5] |
For selected reviews of catalytic dearomatization reactions, see: (a) Pouység, L.; Deffieux, D.; Quideau, S.; Tetrahedron 2010, 66, 2235.
doi: 10.1016/j.tet.2009.12.046 |
|
(b) Roche, S. P.; Porco, J. A. Angew. Chem., Int. Ed. 2011, 50, 4068.
doi: 10.1002/anie.v50.18 |
|
|
(c) Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662.
doi: 10.1002/anie.v51.51 |
|
|
(d) Zheng, C.; You, S.-L. Chem 2016, 1, 830.
doi: 10.1016/j.chempr.2016.11.005 |
|
|
(e) Wu, W.-T.; Zhang, L.; You, S.-L. Chem. Soc. Rev. 2016, 45, 1570.
doi: 10.1039/C5CS00356C |
|
|
(f) Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164.
doi: 10.1039/C5OB02526E |
|
|
(g) Huang, G.; Yin, B. Adv. Synth. Catal. 2019, 361, 405.
doi: 10.1002/adsc.v361.3 |
|
|
(h) Wang, Z. Org. Biomol. Chem. 2020, 18, 4354.
doi: 10.1039/D0OB00818D |
|
|
(i) Zheng, C.; You, S.-L. ACS Cent. Sci. 2021, 7, 432.
doi: 10.1021/acscentsci.0c01651 |
|
|
(j) Liang, R.-X.; Jia, Y.-X. Acc. Chem. Res. 2022, 55, 734.
doi: 10.1021/acs.accounts.1c00781 |
|
| [6] |
(a) Zhao, L.; Li, Z.; Chang, L.; Xu, J.; Yao, H.; Wu, X. Org. Lett. 2012, 14, 2066.
doi: 10.1021/ol300584m pmid: 22463710 |
|
(b) Douki, K.; Ono, H.; Taniguchi, T.; Shimokawa, J.; Kitamura, M.; Fukuyama, T. J. Am. Chem. Soc. 2016, 138, 14578.
doi: 10.1021/jacs.6b10237 pmid: 22463710 |
|
|
(c) Li, X.; Zhou, B.; Yang, R.-Z.; Yang, F.-M.; Liang, R.-X.; Liu, R.-R.; Jia, Y.-X. J. Am. Chem. Soc. 2018, 140, 13945.
doi: 10.1021/jacs.8b09186 pmid: 22463710 |
|
|
(d) Yang, P.; You, S.-L. Org. Lett. 2018, 20, 7684.
doi: 10.1021/acs.orglett.8b03425 pmid: 22463710 |
|
|
(e) Liang, R.-X.; Song, L.-J.; Lu, J.-B.; Xu, W.-Y.; Ding, C.; Jia, Y.-X. Angew. Chem., Int. Ed. 2021, 60, 7412.
doi: 10.1002/anie.v60.13 pmid: 22463710 |
|
|
(f) Han, X.-Q.; Wang, L.; Yang, P.; Liu, J.-L.; Xu, W.-Y.; Zheng, C.; Liang, R.-X.; You, S.-L.; Zhang, J.; Jia, Y.-X. ACS Catal. 2022, 12, 665.
pmid: 22463710 |
|
| [7] |
Yang, P.; Xu, R.-Q.; Zheng, C.; You, S.-L. Chin. J. Chem. 2020, 38, 235.
doi: 10.1002/cjoc.v38.3 |
| [8] |
Cho, C. S.; Uemura, S. J. Organomet. Chem. 1994, 465, 85.
doi: 10.1016/0022-328X(94)87040-3 |
| [9] |
Penn, L.; Shpruhman, A.; Gelman, D. J. Org. Chem. 2007, 72, 3875.
doi: 10.1021/jo070170v |
| [10] |
Lee, A.-L. Org. Biomol. Chem. 2016, 14, 5357.
doi: 10.1039/c5ob01984b pmid: 26529247 |
| [11] |
Gao, S.; Yang, C.; Huang, Y.; Zhao, L.; Wu, X.; Yao, H.; Lin, A. Org. Biomol. Chem. 2016, 14, 840.
doi: 10.1039/C5OB01970B |
| [12] |
For recent asymmetric transformations of indole: (a) Gao, H.; Miao, Y.-H.; Sun, W.-N.; Zhao, R.; Xiao, X.; Hua, Y.-Z.; Jia, S.-K.; Wang, M.-C.; Mei, G.-J. Adv. Sci. 2023, 10, 2305101.
doi: 10.1002/advs.v10.35 pmid: 25316161 |
|
(b) Zhang, H.; Shi, F. Chin. J. Org. Chem. 2022, 42, 3351. (in Chinese)
doi: 10.6023/cjoc202203018 pmid: 25316161 |
|
|
(张洪浩, 石枫, 有机化学, 2022, 42, 3351.)
doi: 10.6023/cjoc202203018 pmid: 25316161 |
|
|
(c) Sheng, F.-T.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Chin. J. Chem. 2022, 40, 2151.
doi: 10.1002/cjoc.v40.18 pmid: 25316161 |
|
|
(d) Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Org. Chem. Front. 2020, 7, 3967.
doi: 10.1039/D0QO01124J pmid: 25316161 |
|
|
(e) Zhang, Y.-C.; Jiang, F.; Shi, F. Acc. Chem. Res. 2020, 53, 425.
doi: 10.1021/acs.accounts.9b00549 pmid: 25316161 |
|
|
(f) Zheng, C.; You, S.-L. Acc. Chem. Res. 2020, 53, 974.
doi: 10.1021/acs.accounts.0c00074 pmid: 25316161 |
|
|
(g) Chen, J.-B.; Jia, Y.-X. Org. Biomol. Chem. 2017, 15, 3550.
doi: 10.1039/C7OB00413C pmid: 25316161 |
|
|
(h) Dalpozzo, R. Chem. Soc. Rev. 2015, 44, 742.
doi: 10.1039/c4cs00209a pmid: 25316161 |
|
|
(i) Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Chem. Soc. Rev. 2010, 39, 4449.
doi: 10.1039/b923063g pmid: 25316161 |
| [1] | Mengmeng Wang, Jun Zhang, Huiying Wang, Biao Ma, Hui-Xiong Dai. Construction of Aza-spiro[4,5]indole Scaffolds via Rhodium-Catalyzed Regioselective C(4)—H Activation of Indole※ [J]. Acta Chimica Sinica, 2022, 80(3): 277-281. |
| [2] | Long Zheng, Lijia Wang, Yong Tang. Intramolecular Ring-opening of Indole-cyclopropanes※ [J]. Acta Chimica Sinica, 2022, 80(3): 255-258. |
| [3] | Linjun Zhan, Wei Hu, Mei Wang, Bin Huang, Ya-Qiu Long. Imidoyl Chloride Mediated One-Pot Synthesis of 3-Electron Withdrawing Group Substituted Indoles [J]. Acta Chimica Sinica, 2021, 79(7): 903-907. |
| [4] | Bo Zhou, Renxiao Liang, Zhongyan Cao, Pinghai Zhou, Yixia Jia. Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles [J]. Acta Chimica Sinica, 2021, 79(2): 176-179. |
| [5] | Danqi Zhang, Yingbo Shao, Hanliang Zheng, Biying Zhou, Xiao-Song Xue. Mechanistic Study on the Bidentate Nitrogen-Ligated Iodine(V) Reagent Promoted Oxidative Dearomatization of Phenols [J]. Acta Chimica Sinica, 2021, 79(11): 1394-1400. |
| [6] | Ren Xiang, Zhang Xiaoping, Wang Yufen, Cao Jingyu, Cheng Yuanyuan, Feng Shouhua, Chen Huanwen. Intramolecular and Intermolecular Methyl Migration of Fenthion Studied by Electrospray Ionization Mass Spectrometry [J]. Acta Chimica Sinica, 2019, 77(4): 358-364. |
| [7] | Liao Fumin, Du Yi, Zhou Feng, Zhou Jian. Au(I)/Chiral Tertiary Amine Catalyzed Tandem Olefination/Asymmetric Cyclization Reaction to Quaternary Spirocyclic Oxindoles [J]. Acta Chim. Sinica, 2018, 76(11): 862-868. |
| [8] | Yan Wen-Guang, Wang Pan, Wang Lijia, Sun Xiu-Li, Tang Yong. Copper Catalyzed[3+2] Annulation of Indoles with 1,1,2,2-Tetrasubstituted Donor-Acceptor Cyclopropanes [J]. Acta Chim. Sinica, 2017, 75(8): 783-787. |
| [9] | Wu Wen-Ting, Zhang Liming, You Shu-Li. Recent Progress on Gold-catalyzed Dearomatization Reactions [J]. Acta Chim. Sinica, 2017, 75(5): 419-438. |
| [10] | Li Wenqiang, Peng Qian, Xie Yujun, Zhang Tian, Shuai Zhigang. Effect of Intermolecular Excited-state Interaction on Vibrationally Resolved Optical Spectra in Organic Molecular Aggregates [J]. Acta Chim. Sinica, 2016, 74(11): 902-909. |
| [11] | Zhang Ling, Zhang Peizhi, Xue Jianfei, Sun Wangbin, Zou Jianping. Manganese Acetate-Mediated Phosphorylation of Indoles [J]. Acta Chim. Sinica, 2016, 74(10): 811-818. |
| [12] | Yin Xiaoping, Xu Pengwei, Dong Kun, Liao Kui, Zhou Feng, Zhou Jian. Ga(OTf)3 Catalyzed Highly Efficient Substitution Reaction of 3-Hydroxyoxindoles Using TMSN3 [J]. Acta Chim. Sinica, 2015, 73(7): 685-689. |
| [13] | Lu Ping, Feng Chao, Loh Teck-Peng. Rh/Ag Bimetallic Catalyzed C-H Bond Olefination of Benzonitriles [J]. Acta Chim. Sinica, 2015, 73(12): 1315-1319. |
| [14] | Duan Dehe, Yin Qin, Wang Shouguo, Gu Qing, You Shuli. Chiral Phosphoric Acid-Catalyzed Asymmetric Cascade Reaction of C(3) Substituted Indoles and Methyl Vinyl Ketone [J]. Acta Chim. Sinica, 2014, 72(9): 1001-1004. |
| [15] | Wang Yuhui, Cao Zhongyan, Niu Yanfei, Zhao Xiaoli, Zhou Jian. Highly Enantioselective Organocatalytic aza-Henry Reaction of Nitroalkanes to N-Boc Isatin Ketimines [J]. Acta Chimica Sinica, 2014, 72(7): 867-872. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||