Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (5): 486-492.DOI: 10.6023/A24020058 Previous Articles Next Articles
Article
赵雨晴a,b, 梁栋b, 贾吉慧d, 余荣民a,b,*( ), 卢灿忠b,c,*(
), 卢灿忠b,c,*( )
)
投稿日期:2024-02-21
发布日期:2024-04-11
基金资助:
Yuqing Zhaoa,b, Dong Liangb, Jihui Jiad, Rongmin Yua,b( ), Can-Zhong Lub,c(
), Can-Zhong Lub,c( )
)
Received:2024-02-21
Published:2024-04-11
Contact:
*E-mail: rongmingyu@fjirsm.ac.cn; czlu@fjirsm.ac.cn
Supported by:Share
Yuqing Zhao, Dong Liang, Jihui Jia, Rongmin Yu, Can-Zhong Lu. Synthesis and Characterization of an Emissive Ag(I) Complex with a D-A Type Ligand Containing Two Electron-withdrawing Groups[J]. Acta Chimica Sinica, 2024, 82(5): 486-492.
| Compound | ||
|---|---|---|
| Distance/nm | Ag1—N1 | 0.2452(4) |
| Ag1—N2 | 0.2295(4) | |
| Ag1—P1 | 0.24142(13) | |
| Ag1—P2 | 0.24334(12) | |
| Angle/(°) | P1—Ag1—P2 | 116.69(4) |
| P1—Ag1—N1 | 103.55(11) | |
| N1—Ag1—N2 | 69.85(15) | |
| N2—Ag1—P2 | 111.25(11) | |
| Dihedral angle/(°) | P1—Ag1—P1/ N1—Ag1—N2 | 78.090(116) |
| Compound | ||
|---|---|---|
| Distance/nm | Ag1—N1 | 0.2452(4) |
| Ag1—N2 | 0.2295(4) | |
| Ag1—P1 | 0.24142(13) | |
| Ag1—P2 | 0.24334(12) | |
| Angle/(°) | P1—Ag1—P2 | 116.69(4) |
| P1—Ag1—N1 | 103.55(11) | |
| N1—Ag1—N2 | 69.85(15) | |
| N2—Ag1—P2 | 111.25(11) | |
| Dihedral angle/(°) | P1—Ag1—P1/ N1—Ag1—N2 | 78.090(116) |
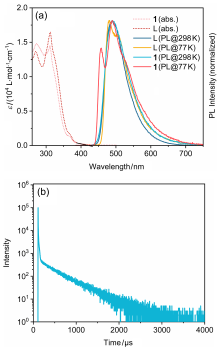

| 298 K | |||||||
|---|---|---|---|---|---|---|---|
| Compound | λmax/ nm | ФPL/ % | ФFa/ % | ФTADFa/ % | τF/ ns | τTADF/ μs | |
| L | 490 | 17.3 | 17.0 | 0.3 | 106.0 | 3272.4 | |
| 1 | 490 | 43.8 | 5.2 | 38.6 | 23.0 | 503.9 | |
| 298 K | |||||||
|---|---|---|---|---|---|---|---|
| Compound | λmax/ nm | ФPL/ % | ФFa/ % | ФTADFa/ % | τF/ ns | τTADF/ μs | |
| L | 490 | 17.3 | 17.0 | 0.3 | 106.0 | 3272.4 | |
| 1 | 490 | 43.8 | 5.2 | 38.6 | 23.0 | 503.9 | |


| [1] |
Lu, J.; Pattengale, B.; Liu, Q.; Yang, S.; Shi, W.; Li, S.; Huang, J.; Zhang, J. J. Am. Chem. Soc. 2018, 140, 13719.
|
| [2] |
Lundberg, P.; Lindh, E. M.; Tang, S.; Edman, L. ACS Appl. Mater. Inter. 2017, 9, 28810.
|
| [3] |
Tang, X.; Cui, L.-S.; Li, H.-C.; Gillett, A. J.; Auras, F.; Qu, Y.-K.; Zhong, C.; Jones, S. T. E.; Jiang, Z.-Q.; Friend, R. H.; Liao, L.-S. Nature Mater. 2020, 19, 1332.
|
| [4] |
Hamze, R.; Peltier, J. L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P. I.; Bertrand, G.; Thompson, M. E. Science 2019, 363, 601.
|
| [5] |
Yuan, P.; Zhang, H.; Zhou, Y.; He, T.; Malola, S.; Gutiérrez-Arzaluz, L.; Li, Y.; Deng, G.; Dong, C.; Huang, R.; Song, X.; Teo, B. K.; Mohammed, O. F.; Häkkinen, H.; Bakr, O. M.; Zheng, N. Aggregate 2024, 5, e475.
|
| [6] |
Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234.
|
| [7] |
Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. J. Am. Chem. Soc. 2012, 134, 14706.
|
| [8] |
Leitl, M. J.; Krylova, V. A.; Djurovich, P. I.; Thompson, M. E.; Yersin, H. J. Am. Chem. Soc. 2014, 136, 16032.
|
| [9] |
Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Nat. Photonics 2014, 8, 326.
|
| [10] |
Im, Y.; Kim, M.; Cho, Y. J.; Seo, J.-A.; Yook, K. S.; Lee, J. Y. Chem. Mater. 2017, 29, 1946.
|
| [11] |
Cai, X.; Su, S. J. Adv. Funct. Mater. 2018, 28, 1802558.
|
| [12] |
Huang, W.; Einzinger, M.; Zhu, T.; Chae, H. S.; Jeon, S.; Ihn, S.-G.; Sim, M.; Kim, S.; Su, M.; Teverovskiy, G.; Wu, T.; Van Voorhis, T.; Swager, T. M.; Baldo, M. A.; Buchwald, S. L. Chem. Mater. 2018, 30, 1462.
|
| [13] |
Wu, T.-L.; Huang, M.-J.; Lin, C.-C.; Huang, P.-Y.; Chou, T.-Y.; Chen-Cheng, R.-W.; Lin, H.-W.; Liu, R.-S.; Cheng, C.-H. Nat. Photonics 2018, 12, 235.
|
| [14] |
Shi, S.; Jung, M. C.; Coburn, C.; Tadle, A.; Sylvinson, M. R. D.; Djurovich, P. I.; Forrest, S. R.; Thompson, M. E. J. Am. Chem. Soc. 2019, 141, 3576.
|
| [15] |
Cai, X. B.; Liang, D.; Yang, M.; Wu, X. Y.; Lu, C. Z.; Yu, R. Chem. Commun. 2022, 58, 8970.
|
| [16] |
Liang, D.; Jia, J.-H.; Cai, X.-B.; Zhao, Y.-Q.; Wang, Z.-Q.; Lu, C.-Z. Inorg. Chem. Front. 2022, 9, 6561.
|
| [17] |
Zhou, T.; Qian, Y.; Wang, H.-J.; Feng, Q.-Y.; Xie, L.-H.; Huang, W. Acta Chim. Sinica 2021, 79, 557 (in Chinese).
doi: 10.6023/A21010009 |
|
(周涛, 钱越, 王宏健, 冯全友, 解令海, 黄维, 化学学报, 2021, 79, 557.)
doi: 10.6023/A21010009 |
|
| [18] |
Wang, Y.-F.; Li, M.; Chen, C.-F. Acta Chim. Sinica 2023, 81, 588 (in Chinese).
|
|
(王银凤, 李猛, 陈传峰, 化学学报, 2023, 81, 588.)
doi: 10.6023/A23040153 |
|
| [19] |
Ge, F.-J.; Zhang, K.-Z.; Cao, Q.-P.; Xu, H.; Zhou, T.; Zhang, W.-H.; Ban, X.-X.; Zhang, X.-B.; Li, N.; Zhu, P. Acta Chim. Sinica 2023, 81, 1157 (in Chinese).
|
|
(葛凤洁, 张开志, 曹清鹏, 徐慧, 周涛, 张文浩, 班鑫鑫, 张晓波, 李娜, 朱鹏, 化学学报, 2023, 81, 1157.)
doi: 10.6023/A23020051 |
|
| [20] |
Liu, Z.-Y.; Rao, J.-F.; Zhu, S.-J.; Wang, B.-Y.; Yu, F.; Feng, Q.-Y.; Xie, L.-H. Acta Chim. Sinica 2023, 81, 820 (in Chinese).
|
|
(刘振宇, 饶俊峰, 祝守加, 王兵洋, 余帆, 冯全友, 解令海, 化学学报, 2023, 81, 820.)
doi: 10.6023/A23040119 |
|
| [21] |
Artem'ev, A. V.; Shafikov, M. Z.; Schinabeck, A.; Antonova, O. V.; Berezin, A. S.; Bagryanskaya, I. Y.; Plusnin, P. E.; Yersin, H. Inorg. Chem. Front. 2019, 6, 3168.
|
| [22] |
Sujith, S.; Nam, E. B.; Lee, J.; Lee, S. U.; Lee, M. H. Inorg. Chem. Front. 2020, 7, 3456.
|
| [23] |
Kim, D.; Coropceanu, V.; Bredas, J. L. J. Am. Chem. Soc. 2011, 133, 17895.
|
| [24] |
Kim, D.; Zhu, L.; Brédas, J.-L. Chem. Mater. 2012, 24, 2604.
|
| [25] |
Jia, J.-H.; Liang, D.; Yu, R.; Chen, X.-L.; Meng, L.; Chang, J.-F.; Liao, J.-Z.; Yang, M.; Li, X.-N.; Lu, C.-Z. Chem. Mater. 2019, 32, 620.
|
| [26] |
Yersin, H.; Czerwieniec, R.; Shafikov, M. Z.; Suleymanova, A. F. Chemphyschem 2017, 18, 3508.
|
| [27] |
Schinabeck, A.; Chen, J.; Kang, L.; Teng, T.; Homeier, H. H. H.; Suleymanova, A. F.; Shafikov, M. Z.; Yu, R.; Lu, C.-Z.; Yersin, H. Chem. Mater. 2019, 31, 4392.
doi: 10.1021/acs.chemmater.9b00671 |
| [28] |
Chen, J.; Teng, T.; Kang, L.; Chen, X. L.; Wu, X. Y.; Yu, R.; Lu, C. Z. Inorg. Chem. 2016, 55, 9528.
pmid: 27652823 |
| [29] |
Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Flechon, C.; Zink, D. M.; Friedrichs, J.; Flugge, H.; Steininger, R.; Gottlicher, J.; Heske, C.; Weinhardt, L.; Brase, S.; So, F.; Baumann, T. Adv. Mater. 2015, 27, 2538.
|
| [30] |
Everly, R. M.; McMillin, D. R. Photochem. Photobiol. 2008, 50, 711.
|
| [31] |
Hsu, C. W.; Lin, C. C.; Chung, M. W.; Chi, Y.; Lee, G. H.; Chou, P. T.; Chang, C. H.; Chen, P. Y. J. Am. Chem. Soc. 2011, 133, 12085.
|
| [32] |
Shafikov, M. Z.; Czerwieniec, R.; Yersin, H. Dalton Trans. 2019, 48, 2802.
doi: 10.1039/c8dt04078h pmid: 30729248 |
| [33] |
Li, Z.; Yang, D.; Han, C.; Zhao, B.; Wang, H.; Man, Y.; Ma, P.; Chang, P.; Ma, D.; Xu, H. Angew. Chem.-Int. Ed. 2021, 60, 14846.
|
| [34] |
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
|
| [1] | Yang Liu, Fengqin Gao, Zhanying Ma, Yinli Zhang, Wuwu Li, Lei Hou, Xiaojuan Zhang, Yaoyu Wang. Co-based Metal-organic Framework for High-efficiency Degradation of Methylene Blue in Water by Peroxymonosulfate Activation [J]. Acta Chimica Sinica, 2024, 82(2): 152-159. |
| [2] | Yinuo Wang, Shiyang Shao, Lixiang Wang. Recent Advances in Multiple Resonance Organic/Polymer Fluorescent Materials with Narrowband Emission★ [J]. Acta Chimica Sinica, 2023, 81(9): 1202-1214. |
| [3] | Huimin Chen, Long Wang, Pan Zhang, Xilin Bai, Guojun Zhou. Investigation on Photoluminescence and Mechanoluminescence of Single Tb3+-doped Intense Green Phosphor [J]. Acta Chimica Sinica, 2023, 81(7): 771-776. |
| [4] | Zhenyu Liu, Junfeng Rao, Shoujia Zhu, Bingyang Wang, Fan Yu, Quanyou Feng, Linghai Xie. Research Progress of Solution-Processed Self-Host Thermally Activated Delayed Fluorescence Materials [J]. Acta Chimica Sinica, 2023, 81(7): 820-835. |
| [5] | Yinfeng Wang, Meng Li, Chuanfeng Chen. Chiral Triptycene-Based Red Thermally Activated Delayed Fluorescence Polymers and Their Organic Light-Emitting Diodes★ [J]. Acta Chimica Sinica, 2023, 81(6): 588-594. |
| [6] | Shaoqin Zhang, Meiqing Li, Zhongjun Zhou, Zexing Qu. Theoretical Study on the Multiple Resonance Thermally Activated Delayed Fluorescence Process [J]. Acta Chimica Sinica, 2023, 81(2): 124-130. |
| [7] | Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※ [J]. Acta Chimica Sinica, 2022, 80(5): 640-646. |
| [8] | Lu Zhou, Jia-Xiong Chen, Shaomin Ji, Wen-Cheng Chen, Yanping Huo. Research Progress of Red Thermally Activated Delayed Fluorescent Materials Based on Quinoxaline [J]. Acta Chimica Sinica, 2022, 80(3): 359-372. |
| [9] | Rong Zhang, Jiangping Liu, Ziyi Zhu, Shumei Chen, Fei Wang, Jian Zhang. Synthesis, Structure and Characterization of Two Ferrocene Functionalized Cadmium Metal Organic Frameworks※ [J]. Acta Chimica Sinica, 2022, 80(3): 249-254. |
| [10] | Jinxu Zhao, Mingshu Zhang, Wenfa Chen, Xiaoming Jiang, Binwen Liu, Guocong Guo. KAg3Ga8S14: An Mid- and Far-infrared Nonlinear Optical Material Exhibiting High Laser-induced Damage Threshold※ [J]. Acta Chimica Sinica, 2022, 80(3): 259-264. |
| [11] | Wentao Wang, Gaochong Zhao, Liu Yang, Yicheng Zhou, Liming Ding. Study on Multimodal Color-switching Anti-counterfeiting Based on Magnetically Responsive Photonic Crystals and Quantum Dots [J]. Acta Chimica Sinica, 2022, 80(12): 1576-1582. |
| [12] | Daolan Xu, Ying Yang, Wentao Fan, Zongbing He, Jiafeng Zou, Lei Feng, Man-Bo Li, Zhikun Wu. Single, Self-Born RP-Au-PR Motif Boosts 19-Fold Photoluminescence Quantum Yield of Metal Nanocluster [J]. Acta Chimica Sinica, 2022, 80(1): 1-6. |
| [13] | Hao-Nan Qin, Zhao-Yang Wang, Shuang-Quan Zang. Photoluminescence and Electrochemical Sensing of Atomically Precise Cu13 Cluster [J]. Acta Chimica Sinica, 2021, 79(8): 1037-1041. |
| [14] | Tao Zhou, Yue Qian, Hongjian Wang, Quanyou Feng, Linghai Xie, Wei Huang. Recent Advances in Substituent Effects of Blue Thermally Activated Delayed Fluorescence Small Molecules [J]. Acta Chimica Sinica, 2021, 79(5): 557-574. |
| [15] | Jing Fang, Wenjuan Zhao, Minghao Zhang, Qianrong Fang. A Novel Amide-functionalized Covalent Organic Framework for Selective Dye Adsorption [J]. Acta Chimica Sinica, 2021, 79(2): 186-191. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||