Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (11): 1356-1362.DOI: 10.6023/A25050166 Previous Articles Next Articles
Article
张士民a, 郝朋飞b,*( ), 于炜玉b, 朱慧慧b, 杨海英a, 沈俊菊b, 付云龙b,*(
), 于炜玉b, 朱慧慧b, 杨海英a, 沈俊菊b, 付云龙b,*( )
)
投稿日期:2025-05-14
发布日期:2025-06-27
基金资助:
Zhang Shimina, Hao Pengfeib,*( ), Yu Weiyub, Zhu Huihuib, Yang Haiyinga, Shen Junjub, Fu Yunlongb,*(
), Yu Weiyub, Zhu Huihuib, Yang Haiyinga, Shen Junjub, Fu Yunlongb,*( )
)
Received:2025-05-14
Published:2025-06-27
Contact:
*E-mail: haopengfei_2015@126.com;yunlongfu@sxnu.edu.cn
Supported by:Share
Zhang Shimin, Hao Pengfei, Yu Weiyu, Zhu Huihui, Yang Haiying, Shen Junju, Fu Yunlong. The Modulation Effect of N-Substituents on Photochromic Properties of Naphthalenediimide-based Coordination Polymers[J]. Acta Chimica Sinica, 2025, 83(11): 1356-1362.
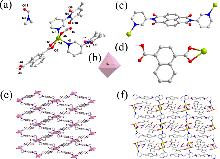

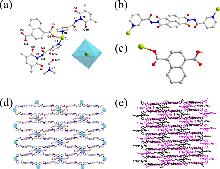

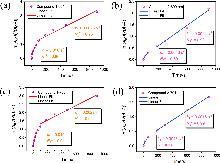

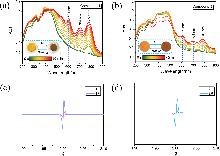

| [30] |
doi: 10.1039/D0CE01733G |
| [31] |
|
| [32] |
|
| [33] |
doi: 10.1016/j.dyepig.2023.111677 |
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
doi: 10.1021/ja209800x |
| [38] |
doi: 10.1016/j.dyepig.2020.108269 |
| [39] |
|
| [40] |
|
| [1] |
|
| [2] |
doi: 10.1016/j.ccr.2021.214304 |
| [3] |
|
| [4] |
|
| [5] |
doi: 10.1016/j.ccr.2022.214918 |
| [6] |
doi: 10.1021/acsami.6b15540 |
| [7] |
|
| [8] |
doi: 10.1039/C9CC05498G |
| [9] |
doi: 10.1039/D0CC06379G |
| [10] |
|
| [11] |
|
| [40] |
(于炜玉, 硕士论文,山西师范大学, 太原, 2022.)
|
| [12] |
doi: 10.1016/j.ccr.2023.215526 |
| [13] |
|
| [14] |
doi: 10.1039/D1CC05070B |
| [15] |
doi: 10.1002/anie.v46:18 |
| [16] |
doi: 10.1021/ja107382x |
| [17] |
doi: 10.1002/chem.v20.24 |
| [18] |
doi: 10.1016/j.dyepig.2020.108941 |
| [19] |
|
| [20] |
doi: 10.1021/acs.inorgchem.3c01613 |
| [21] |
doi: 10.1039/C6DT03105F |
| [22] |
doi: 10.1002/anie.v56.2 |
| [23] |
|
| [24] |
|
| [25] |
doi: 10.1016/j.dyepig.2018.11.008 |
| [26] |
|
| [27] |
doi: 10.1039/C5DT04539H |
| [28] |
doi: 10.1039/C8CE00213D |
| [29] |
doi: 10.1039/C8CE00892B |
| [1] | Xiang-Yu Li, Jia-Kai Li, Shu-Juan Lin, Ming-Sheng Wang, Jing Sun, Zhong-Ning Xu, Guo-Cong Guo. PdZn Alloy Catalysts Modulating the Selectivity of CO Esterification Products: from Dimethyl Oxalate to Dimethyl Carbonate [J]. Acta Chimica Sinica, 2025, 83(3): 206-211. |
| [2] | Cui Xin, Jun Jiang, Zi-Wei Deng, Li-Juan Ou, Wei-Min He. Photoinduced FeCl3‑catalyzed Cross-Dehydrogenative Alkylation of Benzoxazin-2-ones with Alkanes [J]. Acta Chimica Sinica, 2024, 82(11): 1109-1113. |
| [3] | Xun Liu, Hui-Bo Jiang, Kai-Qiang Jing, Zhong-Ning Xu, Guo-Cong Guo. Introduction of Zn2+ Promotes the Catalytic Performance of Pd-based Catalyst for CO Esterification Reaction via Electron Transfer [J]. Acta Chimica Sinica, 2023, 81(7): 691-696. |
| [4] | Junmin Chen, Chengqian Cui, Hanlin Liu, Guodong Li. Study on the Selective Hydrogenation of Quinoline Catalyzed by Composites of Metal-Organic Framework and Pt Nanoparticles※ [J]. Acta Chimica Sinica, 2022, 80(4): 467-475. |
| [5] | Jie Hu, Shanxi Tian. Progresses in the Study of Low-Energy Ion-molecule Reaction Dynamics※ [J]. Acta Chimica Sinica, 2022, 80(4): 535-541. |
| [6] | Ning Xu, Qinglong Qiao, Xiaogang Liu, Zhaochao Xu. Enhancing Brightness and Photostability of Organic Small Molecular Fluorescent Dyes Through Inhibiting Twisted Intramolecular Charge Transfer (TICT)※ [J]. Acta Chimica Sinica, 2022, 80(4): 553-562. |
| [7] | Yu Mohan, Cheng Yuanyuan, Liu Yajun. Mechanistic Study of Oxygenation Reaction in Firefly Bioluminescence [J]. Acta Chimica Sinica, 2020, 78(9): 989-993. |
| [8] | Zhu Qingqing, Song Xiaojun, Deng Zhaoxiang. Tunable Charge Transfer Plasmon at Gold/Copper Heterointerface [J]. Acta Chimica Sinica, 2020, 78(7): 675-679. |
| [9] | Ni Yuxin, Zhang Chenjie, Yuan Yaxian, Xu Minmin, Yao Jianlin. Determination on Origination of Surface Enhanced Raman Scattering Effect on Nano ZnO Substrate [J]. Acta Chim. Sinica, 2019, 77(7): 641-646. |
| [10] | Qi Qige, Yang Chunfan, Xia Ye, Liu Kunhui, Su Hongmei. Photo-induced Electron Transfer between 4-Thiouracil and Tryptophan [J]. Acta Chimica Sinica, 2019, 77(6): 515-519. |
| [11] | Zhang Tong, Yang Zi, Li Hongliang, Zhuang Quanchao, Cui Yanhua. Electrochemical Impedance Spectroscopic Studies of All Solid State battery with Li10GeP2S12 as Electrolyte [J]. Acta Chim. Sinica, 2019, 77(6): 525-532. |
| [12] | Liu Shengwei, Zhao Jianjun, Xu Yiming. Larger Adsorption Effect of Fluoride than Phosphate on Phenol Degradation over the Irradiated Anatase TiO2 and Pt/TiO2 [J]. Acta Chim. Sinica, 2019, 77(4): 351-357. |
| [13] | Qiu Xuan, Shi Liang. Electrical Interplay between Microorganisms and Iron-bearing Minerals [J]. Acta Chim. Sinica, 2017, 75(6): 583-593. |
| [14] | Wang Shaojing, Li Changwei, Li Jin, Chen Bang, Guo Yuan. Novel Coumarin-Based Fluorescent Probes for Detecting Fluoride Ions in Living Cells [J]. Acta Chim. Sinica, 2017, 75(4): 383-390. |
| [15] | Qin Tianyi, Zeng Yi, Chen Jinping, Yu Tianjun, Li Yi. Progress in Organic Fluorescent Thermometers [J]. Acta Chim. Sinica, 2017, 75(12): 1164-1172. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||