Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (11): 1379-1385.DOI: 10.6023/A25050196 Previous Articles Next Articles
Article
雷平a, 苏秦a, 王栋a, Shahid Ali Khanb, 阿布拉江•克依木a,*( )
)
投稿日期:2025-05-29
发布日期:2025-07-11
基金资助:
Lei Pinga, Su Qina, Wang Donga, Shahid Ali Khanb, Ablajan Keyumea,*( )
)
Received:2025-05-29
Published:2025-07-11
Contact:
*E-mail: ablajan209@hotmail.com; Tel.: +86-13999135640
Supported by:Share
Lei Ping, Su Qin, Wang Dong, Shahid Ali Khan, Ablajan Keyume. Catalyst-Free Photochemical Construction of Oxindoles via Electron Donor-Acceptor Complex-Enabled N-Arylacrylamide Cyclization[J]. Acta Chimica Sinica, 2025, 83(11): 1379-1385.
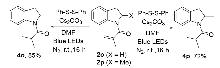
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | none | 56 |
| 2 | A2 instead of A1 | trace |
| 3 | A3 instead of A1 | 35 |
| 4 | A4 instead of A1 | n.r. |
| 5 | A5 instead of A1 | 25 |
| 6 | Cs2CO3 instead of K2CO3 | 70 |
| 7 | DABCO instead of K2CO3 | trace |
| 8 | DBU instead of K2CO3 | 45 |
| 9 | DIPEA instead of K2CO3 | 35 |
| 10 | H2O instead of MeCN | n.r. |
| 11 | DMF instead of MeCN | 90 |
| 12 | DMSO instead of MeCN | trace |
| 13 | 390~395 nm or white light | n.r. |
| 14 | air instead of N2 | 55 |
| 15 | O2 instead of N2 | n.d |
| 16 | without A1 or Cs2CO3 or light irradiation | n.r. |
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | none | 56 |
| 2 | A2 instead of A1 | trace |
| 3 | A3 instead of A1 | 35 |
| 4 | A4 instead of A1 | n.r. |
| 5 | A5 instead of A1 | 25 |
| 6 | Cs2CO3 instead of K2CO3 | 70 |
| 7 | DABCO instead of K2CO3 | trace |
| 8 | DBU instead of K2CO3 | 45 |
| 9 | DIPEA instead of K2CO3 | 35 |
| 10 | H2O instead of MeCN | n.r. |
| 11 | DMF instead of MeCN | 90 |
| 12 | DMSO instead of MeCN | trace |
| 13 | 390~395 nm or white light | n.r. |
| 14 | air instead of N2 | 55 |
| 15 | O2 instead of N2 | n.d |
| 16 | without A1 or Cs2CO3 or light irradiation | n.r. |

| [12] |
(c)
doi: 10.1039/c3sc50810b |
|
(d)
doi: 10.1039/C5CC08253F |
|
|
(e)
doi: 10.1039/C6GC03323G |
|
| [13] |
|
| [14] |
doi: 10.1039/d2sc07078b pmid: 37006691 |
| [15] |
doi: 10.1039/D4GC04554H |
| [16] |
doi: 10.1039/D3OB01970E |
| [17] |
doi: 10.1039/D2QO00205A |
| [1] |
(a)
doi: 10.6023/cjoc202209032 |
|
(赵晓伟, 夏紫琴, 张曼, 周能能, 有机化学, 2022, 42, 3995.)
doi: 10.6023/cjoc202209032 |
|
|
(b)
doi: 10.6023/cjoc202107012 |
|
|
(王弯弯, 张明明, 杨文超, 杨小虎, 有机化学, 2022, 42, 75.)
doi: 10.6023/cjoc202107012 |
|
| [2] |
(a)
doi: 10.1039/C7QO00446J pmid: 34174506 |
|
(b)
doi: 10.1016/j.cclet.2024.110038 pmid: 34174506 |
|
|
(c)
doi: 10.1016/S1872-2067(22)64162-7 pmid: 34174506 |
|
|
(d)
doi: 10.1039/d3sc01072d pmid: 34174506 |
|
|
(e)
doi: 10.6023/cjoc202301023 pmid: 34174506 |
|
|
(岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博, 有机化学, 2023, 43, 3239.)
doi: 10.6023/cjoc202301023 pmid: 34174506 |
|
|
(f)
doi: 10.1016/j.biopha.2021.111842 pmid: 34174506 |
|
| [3] |
(a)
doi: 10.1038/ncomms8204 pmid: 11909708 |
|
(b)
pmid: 11909708 |
|
|
(c)
doi: 10.1021/acs.joc.4c01559 pmid: 11909708 |
|
| [4] |
|
| [5] |
doi: 10.1002/ardp.v353.3 |
| [6] |
doi: 10.1039/C6RA27536B |
| [7] |
|
| [8] |
(a)
doi: 10.1021/acs.chemrev.1c00444 pmid: 17212424 |
|
(b)
pmid: 17212424 |
|
|
(c)
doi: 10.1021/acs.joc.8b00737 pmid: 17212424 |
|
|
(d)
doi: 10.1016/j.ejmech.2020.112752 pmid: 17212424 |
|
| [9] |
|
|
(王丽丽, 张洲, 王廷良, 王兴兰, 毛远湖, 张吉泉, 有机化学, 2024, 44, 2898.)
|
|
| [10] |
(a)
doi: 10.1002/cjoc.v42.20 |
|
(b)
doi: 10.1021/acscatal.3c05994 |
|
|
(c)
doi: 10.1021/acscatal.2c04128 |
|
|
(d)
doi: 10.6023/cjoc202407017 |
|
|
(谭永波, 舒洪波, 黄华文, 有机化学, 2025, 45, 2086.)
doi: 10.6023/cjoc202407017 |
|
|
(e)
|
|
| [11] |
(a)
doi: 10.1021/acs.joc.5b02433 pmid: 26751624 |
|
(b)
doi: 10.1039/C7CC08704G pmid: 26751624 |
|
|
(c)
doi: 10.1021/acscatal.8b00554 pmid: 26751624 |
|
|
(d)
doi: 10.1021/ol403739w pmid: 26751624 |
|
|
(e)
doi: 10.6023/cjoc202211003 pmid: 26751624 |
|
| [18] |
doi: 10.1021/acs.orglett.2c00867 |
| [19] |
doi: 10.1039/D3SC01867A |
| [20] |
doi: 10.1021/acs.orglett.0c02907 |
| [11] |
(任志军, 罗维纬, 周俊, 有机化学, 2023, 43, 2026).
pmid: 26751624 |
|
(f)
doi: 10.1021/acscatal.1c00649 pmid: 26751624 |
|
|
(g)
doi: 10.1021/acs.orglett.1c01698 pmid: 26751624 |
|
| [12] |
(a)
doi: 10.1021/acs.joc.8b02277 |
|
(b)
doi: 10.1039/C4GC00231H |
| [1] | Bo Zhou, Renxiao Liang, Zhongyan Cao, Pinghai Zhou, Yixia Jia. Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles [J]. Acta Chimica Sinica, 2021, 79(2): 176-179. |
| [2] | Liao Fumin, Du Yi, Zhou Feng, Zhou Jian. Au(I)/Chiral Tertiary Amine Catalyzed Tandem Olefination/Asymmetric Cyclization Reaction to Quaternary Spirocyclic Oxindoles [J]. Acta Chim. Sinica, 2018, 76(11): 862-868. |
| [3] | Yin Xiaoping, Xu Pengwei, Dong Kun, Liao Kui, Zhou Feng, Zhou Jian. Ga(OTf)3 Catalyzed Highly Efficient Substitution Reaction of 3-Hydroxyoxindoles Using TMSN3 [J]. Acta Chim. Sinica, 2015, 73(7): 685-689. |
| [4] | Wang Yuhui, Cao Zhongyan, Niu Yanfei, Zhao Xiaoli, Zhou Jian. Highly Enantioselective Organocatalytic aza-Henry Reaction of Nitroalkanes to N-Boc Isatin Ketimines [J]. Acta Chimica Sinica, 2014, 72(7): 867-872. |
| [5] | Li Bo, Zhou Rui, He Gu, Guo Li, Huang Wei. Molecular Docking, QSAR and Molecular Dynamics Simulation on Spiro-oxindoles as MDM2 Inhibitors [J]. Acta Chimica Sinica, 2013, 71(10): 1396-1403. |
| [6] | Sun Xunhao, Peng Jing, Zhang Shuyang, Zhou Qingqing, Dong Lin, Chen Yingchun. Asymmetric Allylic Alkylation of Cyclic N-Sulfonylimines with Morita-Baylis-Hillman Carbonates of Isatins [J]. Acta Chimica Sinica, 2012, 70(16): 1682-1685. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||