Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (11): 1451-1462.DOI: 10.6023/A25060204 Previous Articles
Review
周泓宇a, 王俪颖a, 赵宇a, 吴泽轩b, 李国鹏b, 李念武b,*( ), 楚攀a,*(
), 楚攀a,*( )
)
投稿日期:2025-06-05
发布日期:2025-08-28
作者简介: |
周泓宇, 男, 助理研究员, 于2017年获吉林大学学士学位; 2022年获吉林大学博士学位; 2023年加入中石油深圳新能源研究院有限公司, 目前主要研究方向为新型储能材料与电芯开发. |
 |
李念武, 男, 硕士生导师, 北京化工大学化学工程学院副教授, 中国颗粒协会青年理事. 于2014年南京航空航天大学获得工学博士学位, 2014年~2016年在中科院化学所从事博士后研究工作, 2017年~2018年在北京纳米能源与系统研究所任助理研究员. 目前主要研究方向为锂/钠电池负极和固态电池. 在Adv. Mater. 等期刊上发表论文80余篇, SCI总引用10000余次, H-index为40, 入选2023年科睿唯安全球高被引学者, 2024全球前2%顶尖科学家年度影响力榜. 主持国家自然科学基金青年基金和面上基金项目, 作为骨干参与国家重点研发计划. 已授权国内发明专利10项, 国际PCT专利1项. |
 |
楚攀, 中石油深圳新能源研究院有限公司高级工程师, 中国化学与物理电源行业协会储能应用分会专家委员, 中国石油学会新能源专业委员会专家委员, 中国工业节能与清洁生产协会电池回收与再利用分会专家委员, 储能领跑者联盟专家技术顾问; 长期从事能源高效利用及节能理论、新能源发电, 大规模储能技术等领域的相关工程技术研究; 参与了国内首个大规模储能方向的“国家863课题”, 主导并完成了多个集团公司的储能科技项目和储能工程项目, 近年来参与的储能项目超过50个, 在国内外权威期刊上发表SCI/EI检索论文30余篇, 并在储能领域获得了专有专利技术30余项. |
基金资助:
Zhou Hongyua, Wang Liyinga, Zhao Yua, Wu Zexuanb, Li Guopengb, Li Nianwub,*( ), Chu Pana,*(
), Chu Pana,*( )
)
Received:2025-06-05
Published:2025-08-28
Contact:
*E-mail: linianwu@mail.buct.edu.cn;chupan@petrochina.com.cn
Supported by:Share
Zhou Hongyu, Wang Liying, Zhao Yu, Wu Zexuan, Li Guopeng, Li Nianwu, Chu Pan. Hydrogen Bond Chemistry Enabled Key Materials for High-Energy-Density Lithium Batteries[J]. Acta Chimica Sinica, 2025, 83(11): 1451-1462.


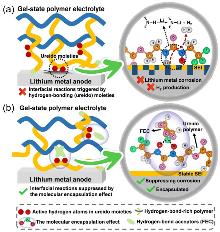





| [1] |
doi: 10.1002/aenm.v12.26 |
| [2] |
doi: 10.1002/adfm.v33.49 |
| [3] |
|
| [4] |
doi: 10.1016/j.esci.2022.02.008 |
| [5] |
doi: 10.1002/adma.v28.9 |
| [6] |
doi: 10.1002/anie.v57.6 |
| [7] |
doi: 10.1002/adma.v31.50 |
| [8] |
doi: 10.1039/D3CS00705G |
| [9] |
doi: 10.1021/ja01315a102 |
| [10] |
doi: 10.1021/ja01452a015 |
| [11] |
doi: 10.1126/science.1242603 |
| [12] |
doi: 10.1002/advs.v11.22 |
| [13] |
doi: 10.1039/D3MH00236E |
| [14] |
doi: 10.1007/s11426-023-1559-9 |
| [15] |
|
| [16] |
doi: 10.1039/D4EE02827A |
| [17] |
doi: 10.1002/adfm.v32.36 |
| [18] |
doi: 10.1002/adfm.v31.3 |
| [19] |
doi: 10.6023/A24050160 |
|
(郑坤贵, 刘君珂, 胡轶旸, 尹祖伟, 周尧, 李君涛, 孙世刚, 化学学报, 2024, 82, 833.)
doi: 10.6023/A24050160 |
|
| [20] |
|
| [21] |
|
| [22] |
doi: 10.1016/j.esci.2024.100265 |
| [23] |
doi: 10.1002/aenm.v10.29 |
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
doi: 10.1016/j.esci.2023.100180 |
| [28] |
doi: 10.1039/C5RA24338F |
| [29] |
doi: 10.1016/j.cej.2021.130844 |
| [30] |
doi: 10.1002/adfm.v32.34 |
| [31] |
doi: 10.1016/j.cej.2022.136347 |
| [32] |
doi: 10.1016/j.cej.2020.124404 |
| [33] |
doi: 10.1016/j.cej.2022.139128 |
| [34] |
doi: 10.1016/j.esci.2024.100268 |
| [35] |
doi: 10.1038/s41557-024-01585-y |
| [36] |
doi: 10.1002/anie.v60.19 |
| [37] |
|
| [38] |
doi: 10.1038/s41467-024-46186-y |
| [39] |
doi: 10.1021/jacs.4c09027 |
| [40] |
doi: 10.1002/adfm.v33.1 |
| [41] |
doi: 10.1002/cnl2.v3.3 |
| [42] |
doi: 10.1002/advs.v12.19 |
| [43] |
|
| [44] |
doi: 10.1002/advs.v9.4 |
| [45] |
doi: 10.1021/jacs.3c00647 |
| [46] |
doi: 10.1002/advs.v10.3 |
| [47] |
doi: 10.1039/D4EE02218A |
| [48] |
doi: 10.1002/adma.v35.45 |
| [49] |
doi: 10.1002/adfm.v35.1 |
| [50] |
doi: 10.1002/adma.v35.22 |
| [51] |
doi: 10.1016/j.cej.2024.150888 |
| [52] |
|
| [53] |
doi: 10.1002/anie.v60.30 |
| [54] |
doi: 10.1016/j.esci.2024.100278 |
| [55] |
doi: 10.1002/cnl2.v3.4 |
| [56] |
doi: 10.1016/j.esci.2024.100247 |
| [57] |
doi: 10.20517/energymater |
| [58] |
doi: 10.6023/A24090267 |
|
(陈博文, 徐克, 陈琪, 陈立桅, 化学学报, 2024, 82, 1209.)
doi: 10.6023/A24090267 |
|
| [59] |
|
| [60] |
doi: 10.1002/advs.v11.38 |
| [61] |
doi: 10.1016/j.cej.2022.134775 |
| [62] |
|
| [63] |
doi: 10.1039/D3TA01968C |
| [64] |
doi: 10.1039/D1TA05396E |
| [65] |
doi: 10.1039/C8TA01907J |
| [66] |
doi: 10.1002/adfm.v34.16 |
| [67] |
|
| [68] |
doi: 10.1021/jacs.2c06512 |
| [69] |
|
| [70] |
doi: 10.1002/adma.v32.37 |
| [71] |
|
| [72] |
doi: 10.1016/j.jpowsour.2023.233806 |
| [73] |
doi: 10.1002/adfm.v35.38 |
| [74] |
doi: 10.1016/j.electacta.2017.10.037 |
| [75] |
doi: 10.1002/adma.v36.49 |
| [76] |
doi: 10.1002/adfm.v33.45 |
| [77] |
doi: 10.1039/D2EE02904A |
| [78] |
doi: 10.1016/j.nanoen.2022.107105 |
| [79] |
doi: 10.1021/acsami.3c03771 |
| [80] |
doi: 10.1002/adfm.v35.26 |
| [81] |
|
| [82] |
doi: 10.1021/acsenergylett.1c02719 |
| [83] |
doi: 10.1016/j.jpowsour.2019.227548 |
| [84] |
doi: 10.1016/j.cej.2023.146400 |
| [85] |
doi: 10.1016/j.cej.2023.147559 |
| [86] |
doi: 10.1016/j.jpowsour.2021.230947 |
| [87] |
doi: 10.1039/D4TA06151A |
| [88] |
doi: 10.1039/D4TA05967K |
| [89] |
doi: 10.1016/j.esci.2024.100266 |
| [90] |
|
| [91] |
doi: 10.1016/j.actphy.2025.100094 |
| [92] |
doi: 10.1039/D1TA02297K |
| [93] |
|
| [94] |
doi: 10.1002/adfm.v34.38 |
| [95] |
|
| [96] |
doi: 10.1039/D4TA05328A |
| [97] |
|
| [98] |
|
| [99] |
doi: 10.1021/acsami.2c12128 |
| [100] |
|
| [101] |
doi: 10.1002/adfm.v34.39 |
| [102] |
|
| [103] |
|
| [104] |
doi: 10.1021/acsenergylett.2c00461 |
| [1] | Xiaogai Peng, Zhubin Hu, Haitao Sun. Theoretical Study on Dihydrogen Bond Interaction Dominated Dodecaborate-Solvent Molecular Clusters [J]. Acta Chimica Sinica, 2025, 83(5): 510-517. |
| [2] | Xiaomao Tian, Yuequn Lin, Han Zhu, Chao Huang, Bixue Zhu. Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles [J]. Acta Chimica Sinica, 2023, 81(1): 20-28. |
| [3] | Chuanzhi Liu, Fen Li, Jingjing Wang, Xiaolu Zhao, Tingmei Zhang, Xin Huang, Mengli Wu, Zhiyuan Hu, Xinming Liu, Zhanting Li. Self-assembly of Supramolecular Planar Macrocycle Driven by Intermolecular Halogen Bonding [J]. Acta Chimica Sinica, 2022, 80(10): 1365-1368. |
| [4] | Jianxin Tian, Huijuan Guo, Jing Wan, Guixian Liu, Huijuan Yan, Rui Wen, Lijun Wan. In Situ/Operando Advances of Electrode Processes in Solid-state Lithium Batteries [J]. Acta Chimica Sinica, 2021, 79(10): 1197-1213. |
| [5] | Lin Zu-Jin, Cao Rong. Porous Hydrogen-bonded Organic Frameworks (HOFs): Status and Challenges [J]. Acta Chimica Sinica, 2020, 78(12): 1309-1335. |
| [6] | Qin Xiaozhuan, Wang Xinchao, Feng Dandan, He Jiabei, Zheng Liping, Wang Yong, Xie Guanghui, Li Jingjing, Ding Ge. Study on Properties of Excited-state Intermolecular Proton Transfer (ESPT) Reaction Dendrite Containing Benzidine Fragments of Organic Chromophore [J]. Acta Chim. Sinica, 2019, 77(8): 751-757. |
| [7] | Zhang Yaping, Yang Zhen, Li Qikai, He Yuanhang. Carbon-rich Clusters and Graphite-like Structure Formation during Early Detonation of 2,4,6-Trinitrotoluene (TNT) via Molecular Dynamics Simulation [J]. Acta Chim. Sinica, 2018, 76(7): 556-563. |
| [8] | Zhu Xuewei, Cui Xiaoyu, Cai Wensheng, Shao Xueguang. Temperature Dependent Near Infrared Spectroscopy for Understanding the Hydrogen Bonding of Amines [J]. Acta Chim. Sinica, 2018, 76(4): 298-302. |
| [9] | Zhang Yu-Jian, Xie Bin, Jiang Tao. Preparation and Properties of Crown Ethers Containing Amphiphilic Copolymer Nano-aggregates [J]. Acta Chim. Sinica, 2016, 74(9): 752-757. |
| [10] | Zhao Qing, Qi Boyu, Wang Baojin, Chen Feiwu. Theoretical Investigation on Weak Interactions of HXeBr with Benzene and Its Derivatives [J]. Acta Chim. Sinica, 2016, 74(3): 285-292. |
| [11] | Luo Fei, Zheng Jieyun, Chu Geng, Liu Bonan, Zhang Sulin, Li Hong, Chen Liquan. Self-healing Behavior of High Capacity Metal Gallium Thin Film and Powder as Anode Material for Li-ion Battery [J]. Acta Chim. Sinica, 2015, 73(8): 808-814. |
| [12] | Zong Qianying, Geng Huimin, Wang Lu, Ye Lin, Zhang Aiying, Shao Ziqiang, Feng Zengguo. A Study on Synthesis and Gelation Capability of Fmoc and Boc Disubstituted Cyclo(L-Lys-L-Lys)s [J]. Acta Chim. Sinica, 2015, 73(5): 423-430. |
| [13] | Zhang Qi, Fang Hongxia, Zhang Huili, Qin Dan, Hong Zhi, Du Yong. Co-crystal between Nitrofurantion and Urea Investigated by Terahertz Spectroscopy and Density Functional Theory [J]. Acta Chim. Sinica, 2015, 73(10): 1069-1073. |
| [14] | Zhang Qianhui, Wang Yang, Liu Cui, Yang Zhongzhi. Investigation of Base Pairs Containing 7,8-Dihydro-8-oxoguanine by Quantum Chemistry [J]. Acta Chimica Sinica, 2014, 72(8): 956-962. |
| [15] | Wang Xinhua, Feng Li, Cao Zexing. Structure, Energetics and Vibrational Frequency Shifts of Water Molecules Confined Inside Single-walled Carbon Nanotubes:A DFT Study [J]. Acta Chimica Sinica, 2014, 72(4): 487-494. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||