Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (11): 1089-1098.DOI: 10.6023/A19080296 Previous Articles Next Articles
Review
金交羽ab, 严小璇b, 刘亚平b, 蓝文贤b, 王春喜b, 许斌a*( ), 曹春阳b*(
), 曹春阳b*( )
)
投稿日期:2019-08-07
发布日期:2019-08-15
通讯作者:
许斌,曹春阳
E-mail:xubin@t.shu.edu.cn;ccao@mail.sioc.ac.cn
作者简介:金交羽, 女, 上海大学和中科院上海有机所2017级联合培养在读硕士研究生, 主要研究方向为APOBEC3胞嘧啶脱氨基化酶的结构与机理|许斌, 男, 上海大学化学系教授、博导. 2000年获中国科学院上海有机化学研究所理学博士(导师:麻生明院士); 2000~2002年, 在美国国立卫生研究院(NIH)进行博士后研究, 从事基于嘌呤受体的激动剂和拮抗剂的设计与合成(导师: K. A. Jaconson). 2002~2005年, 于美国VivoQuest公司担任高级研究员, 从事抗丙型肝炎药物的设计和合成; 2005年底加入上海大学化学系; 2007年入选上海市“浦江人才”计划.曾获宝钢优秀教师奖、ACP Advanced Research Network Lectureship Award、Asia Core Program Lectureship Award等荣誉.研究方向: (1)惰性化学键可控转化; (2)新型药物分子的设计与合成|曹春阳, 男, 1970年8月出生. 2001年博士毕业于中国科学院上海有机化学研究所, 随后在美国约翰霍普金斯大学医学院做博士后与研究助理, 2005年12月至2006年08月, 转至美国Salk生物研究院结构生物学中心做助理研究员.现任中国科学院上海有机化学研究所“百人计划”研究员, 是上海市“浦江计划”获得者.研究方向:以健康与疾病导向的、以NMR为主要技术手段的化学生物学与结构生物学.内容包括: (1)与癌症或者HIV病毒感染等相关蛋白质与核酸特异性作用机制与功能研究; (2)以与癌症或者HIV病毒感染等相关核酸或蛋白质为靶标, 进行药物分子设计与筛选; (3)基于核磁共振波谱学的蛋白质高效表达新技术新方法研究.
基金资助:
Jin Jiaoyuab, Yan Xiaoxuanb, Liu Yapingb, Lan Wenxianb, Wang Chunxib, Xu Bina*( ), Cao Chunyangb*(
), Cao Chunyangb*( )
)
Received:2019-08-07
Published:2019-08-15
Contact:
Xu Bin,Cao Chunyang
E-mail:xubin@t.shu.edu.cn;ccao@mail.sioc.ac.cn
Share
Jin Jiaoyu, Yan Xiaoxuan, Liu Yaping, Lan Wenxian, Wang Chunxi, Xu Bin, Cao Chunyang. Recent Advances in the Structural Studies on Cytosine Deaminase APOBEC3 Family Members and Their Nucleic Acid Complexes[J]. Acta Chimica Sinica, 2019, 77(11): 1089-1098.
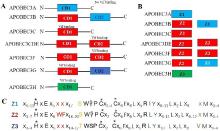








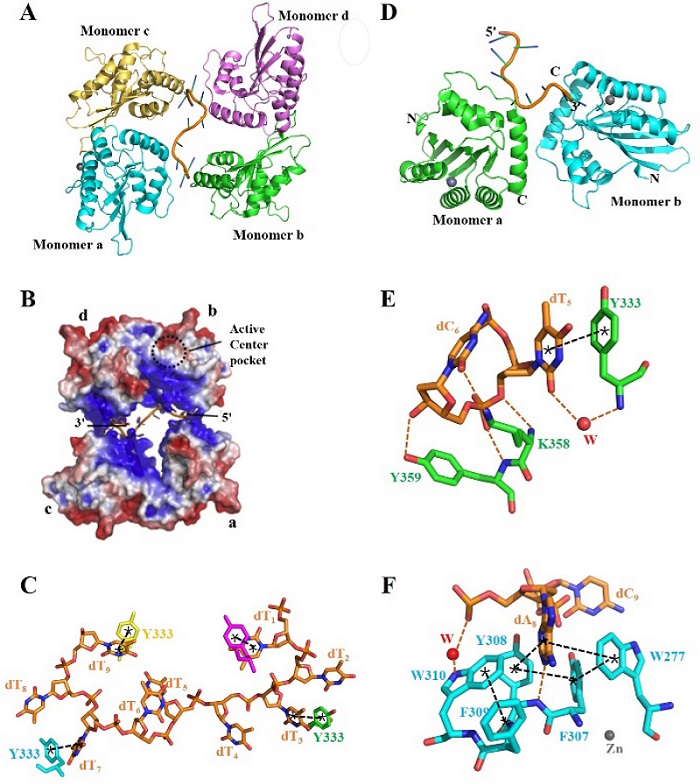




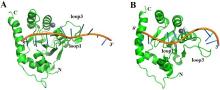

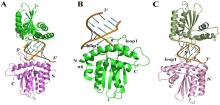

| [1] |
Goila-Gaur R.; Strebel K. Retrovirology 2008, 5, 51.
doi: 10.1186/1742-4690-5-51 |
| [2] |
Larue R. S.; Andresdottir V.; Blanchard Y.; Conticello S. G.; Derse D.; Emerman M.; Greene W. C.; Jonsson S. R.; Landau N. R.; Lochelt M.; Malik H. S.; Malim M. H.; Munk C.; O'Brien S. J.; Pathak V. K.; Strebel K.; Wain-Hobson S.; Yu X. F.; Yuhki N.; Harris R. S. J. Virol. 2009, 83, 494.
doi: 10.1128/JVI.01976-08 |
| [3] |
Wedekind J. E. Trends Genet. 2003, 19, 207.
doi: 10.1016/S0168-9525(03)00054-4 |
| [4] |
Harris R. S.; Liddament M. T. Nat. Rev. Immunol. 2004, 4, 868.
doi: 10.1038/nri1489 |
| [5] |
Kitamura S.; Ode H.; Nakashima M. Nat. Struct. Mol. Biol. 2012, 19, 1005.
doi: 10.1038/nsmb.2378 |
| [6] |
Mitra M.; Hercik K.; Byeon I. J. L. Nucleic Acids Res. 2014, 42, 1095.
doi: 10.1093/nar/gkt945 |
| [7] | Chen K. M.; Harjes E.; Gross P. J.; Fahmy A.; Lu Y.; Shindo K. Seibutsu Butsuri 2008, 48, 116 |
| [8] |
Burns M. B.; Lackey L.; Carpenter M. A.; Rathore A.; Land A. M.; Leonard B. Nature 2013, 494, 366.
doi: 10.1038/nature11881 |
| [9] |
Shi K.; Carpenter M. A.; Kurahashi K.; Harris R. S.; Aihara H. J. Biol. Chem. 2015, 290, 28120.
doi: 10.1074/jbc.M115.679951 |
| [10] |
Xiao X.; Yang H.; Arutiunian V.; Fang Y.; Chen X. S. Nucleic Acids Res. 2017, 45, 7494.
doi: 10.1093/nar/gkx362 |
| [11] |
Nathans R.; Cao H.; Sharova N.; Ali A.; Sharkey M.; Stranska R. Nat. Biotechnol. 2008, 26, 1187.
doi: 10.1038/nbt.1496 |
| [12] |
Dang Y.; Wang X.; Esselman W. J.; Zheng Y. H. J. Virol. 2006, 80, 10522.
doi: 10.1128/JVI.01123-06 |
| [13] |
Stanley B. J.; Ehrlich E. S.; Short L.; Yu Y.; Xiong Y. J. Virol. 2008, 82, 8656.
doi: 10.1128/JVI.00767-08 |
| [14] |
Seplyarskiy V. B.; Andrianova M. A.; Bazykin G. A. Genome Res. 2017, 27, 175.
doi: 10.1101/gr.210336.116 |
| [15] |
Zheng Y. H.; Irwin D.; Kurosu T. J. Virol. 2004, 78, 6073.
doi: 10.1128/JVI.78.11.6073-6076.2004 |
| [16] |
Byeon I. J. L.; Ahn J.; Mitra M.; Byeon C. H.; Hercík K.; Hritz J. Nat. Commun. 2013, 4, 1890.
doi: 10.1038/ncomms2883 |
| [17] | Chelico L.; Prochnow C.; Erie D. A. J. Biol. Chem. 2010, 283, 16195 |
| [18] |
Guo Y.; Dong L.; Qiu X.; Wang Y.; Zhang B.; Liu H. Nature 2014, 505 7482 229.
doi: 10.1038/nature12884 |
| [19] |
Bohn M. F. Structure 2013, 21, 1042.
doi: 10.1016/j.str.2013.04.010 |
| [20] |
Siu K. K.; Sultana A.; Azimi F. Nat. Commun. 2013, 4, 2593.
doi: 10.1038/ncomms3593 |
| [21] |
Matsui M.; Shindo K.; Izumi T.; Io K.; Shinohara M.; Komano J.; Kobayashi M.; Kadowaki N.; Harris R. S.; Takaori-Kondo A. Virol. J. 2014, 11, 122.
doi: 10.1186/1743-422X-11-122 |
| [22] |
Kouno T.; Luengas E. M.; Shigematsu M. Nat. Struct. Mol. Biol. 2015, 22 6 485.
doi: 10.1038/nsmb.3033 |
| [23] |
Klarmann G. J. J. Biol. Chem. 2003, 278, 7902.
doi: 10.1074/jbc.M207223200 |
| [24] |
Furukawa A.; Nagata T.; Matsugami A. Nucleic Acids Symp. Ser. 2009, 53, 87.
doi: 10.1093/nass/nrp044 |
| [25] | Mangeat B.; Turelli P.; Caron G.; Friedli M.; Perrin L.; Trono D. Nature 2003, 4244, 99 |
| [26] |
Peng G. J. Exp. Med. 2006, 203, 41.
doi: 10.1084/jem.20051512 |
| [27] |
Chelico L.; Pham P.; Calabrese P.; Goodman M. F. Nat. Struct. Mol. Biol. 2006, 13, 392.
doi: 10.1038/nsmb1086 |
| [28] |
Harjes E.; Gross P. J.; Chen K. M.; Lu Y.; Shindo K. J. Mol. Biol. 2009, 389, 819.
doi: 10.1016/j.jmb.2009.04.031 |
| [29] | Wichroski M. J. K. Ichiyama; T. M. Rana. J. Biol. Chem. 2005, 280, 8387 |
| [30] |
Sheehy A. M.; Gaddis N. C.; Choi J. D.; Malim M. H. Nature 2002, 418, 646.
doi: 10.1038/nature00939 |
| [31] |
Bennett R. P.; Presnyak V.; Wedekind J. E.; Smith H. C. J. Biol. Chem. 2008, 283, 7320.
doi: 10.1074/jbc.M708567200 |
| [32] |
Lu X.; Zhang T. L.; Xu Z. J. Biol. Chem. 2015, 290, 4010.
doi: 10.1074/jbc.M114.624262 |
| [33] |
Dang Y.; Siew L. M.; Wang X. J.; Han Y. X.; Lampen R.; Zheng Y. H. J. Biol. Chem. 2008, 283, 11606.
doi: 10.1074/jbc.M707586200 |
| [34] |
Jarmuz A.; Chester A.; Bayliss J.; Gisbourne J.; Dunham I.; Scott J. Genomics 2002, 79, 285.
doi: 10.1006/geno.2002.6718 |
| [35] |
Holden L. G.; Prochnow C.; Chang Y. P. Nature 2008, 456, 121.
doi: 10.1038/nature07357 |
| [36] |
Olson M. E.; Li M.; Harris R. S. Chem. Med. Chem. 2013, 8, 112.
doi: 10.1002/cmdc.201200411 |
| [37] |
Jager S.; Kim D. Y.; Hultquist J. F.; Shindo K.; Larue R. S.; Kwon E. Nature 2012, 481, 371.
doi: 10.1038/nature10693 |
| [38] |
Mehle A.; Strack B.; Ancuta P.; Zhang C.; Mcpike M.; Gabuzda D. J. Biol. Chem. 2004, 279, 7792.
doi: 10.1074/jbc.M313093200 |
| [39] |
Marcsisin S. R.; Engen J. R. J. Mol. Biol. 2010, 402, 892.
doi: 10.1016/j.jmb.2010.08.026 |
| [40] |
Bergeron J. R.; Huthoff H.; Veselkov D. A.; Beavil R. L.; Sanderson M. R. PLoS Pathog. 2010, 6, e1000925.
doi: 10.1371/journal.ppat.1000925 |
| [41] |
Reingewertz T. H.; Shalev D. E.; Friedler A. Protein Pept. Lett. 2010, 17, 988.
doi: 10.2174/092986610791498876 |
| [42] |
Liddament M. T.; Brown W. L.; Schumacher A. J.; Harris R. S. Curr. Biol. 2004, 14, 1385.
doi: 10.1016/j.cub.2004.06.050 |
| [43] |
Lu Z.; Bergeron J. R. C.; AtkinsLu Z.; Bergeron J. R. C.; Atkinson R. A.; Schaller T.; Veselkov D. A.; Oregioni A. Open. Biol. 2013, 3, 130100.
doi: 10.1098/rsob.130100 |
| [44] |
Luo K.; Xiao Z.; Ehrlich E.; Yu Y.; Liu B.; Zheng S. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 11444.
doi: 10.1073/pnas.0502440102 |
| [45] |
Stenglein M. D.; Burns M. B.; Li M.; Lengyel J.; Harris R. S. Nat. Struct. Mol. Biol. 2010, 17, 222.
doi: 10.1038/nsmb.1744 |
| [46] |
Chelico L.; Pham P.; Calabrese P.; Goodman M. F. Nat. Struct. Mol. Biol. 2006, 13, 392.
doi: 10.1038/nsmb1086 |
| [47] |
Ronsard L.; Raja R.; Panwar V.; Saini S.; Mohankumar K.; Sridharan S. Sci. Rep. 2015, 5, 15438.
doi: 10.1038/srep15438 |
| [48] |
Yamanaka S.; Balestra M. E.; Ferrell L. D. Proc. Nat. Acad. Sci. U. S. A. 1995, 92, 8483.
doi: 10.1073/pnas.92.18.8483 |
| [49] |
Navarro F.; Bollman B.; Chen H. Virology 2005, 333, 374.
doi: 10.1016/j.virol.2005.01.011 |
| [50] |
Kouno T.; Silvas T. V.; Hilbert B. J.; Shandilya S. M. D.; Bohn M. F.; Kelch B. A.; Royer W. E.; Somasundaran M.; Kurt Yilmaz N.; Matsuo H.; Schiffer C. A. Nat. Commun. 2017, 8, 15024.
doi: 10.1038/ncomms15024 |
| [51] |
Shi K.; Carpenter M. A.; Banerjee S.; Shaban N. M.; Kurahashi K.; Salamango D. J.; McCann J. L.; Starrett G. J.; Duffy J. V.; Demir O.; Amaro R. E.; Harki D. A.; Harris R. S.; Aihara H. Nat. Struct Mol. Biol. 2017, 24, 131.
doi: 10.1038/nsmb.3344 |
| [52] |
Fang Y.; Xiao X.; Li S.; Wolfe A.; Chen X. S. J. Mol. Biol. 2018, 430, 87.
doi: 10.1016/j.jmb.2017.11.007 |
| [53] |
Cheng C.; Zhang T.; Wang C. X.; Lan W.; Ding J.; Cao C. Y. Chin. J. Chem. 2018, 36, 1241.
doi: 10.1002/cjoc.201800508 |
| [54] |
Xiao X.; Li S. X.; Yang H.; Chen X. S. Nat. Commun. 2016, 7, 12193.
doi: 10.1038/ncomms12193 |
| [55] |
Maiti A.; Myint W.; Kanai T.; Delviks-Frankenberry K.; Sierra Rodriguez C.; Pathak V. K.; Schiffer C. A.; Matsuo H. Nat. Commun. 2018, 9, 2460.
doi: 10.1038/s41467-018-04872-8 |
| [56] |
Yan X. X.; Lan W. X.; Wang C. X.; Cao C. Y. Chem. Asian J. 2019, 14, 2235.
doi: 10.1002/asia.201900480 |
| [57] |
Bohn J. A.; Thummar K.; York A.; Raymond A.; Brown W. C.; Bieniasz P. D.; Hatziioannou T.; Smith J. L. Nat. Commun. 2017, 8, 1021.
doi: 10.1038/s41467-017-01309-6 |
| [58] |
Nadine M. S.; Shi K.; Lauer K. V.; Brown W. L.; Aihara H.; Harris R. S. Mol. Cell 2018, 69, 75.
doi: 10.1016/j.molcel.2017.12.010 |
| [59] |
Matsuoka T.; Nagae T.; Ode H.; Awazu H.; Kurosawa T.; Hamano A.; Matsuoka K.; Hachiya A.; Imahashi M.; Yokomaku Y.; Watanabe N.; Iwatani Y. Nucleic Acids Res. 2018, 46, 10368
doi: 10.1093/nar/gky676 |
| [1] | Yang Liu, Fengqin Gao, Zhanying Ma, Yinli Zhang, Wuwu Li, Lei Hou, Xiaojuan Zhang, Yaoyu Wang. Co-based Metal-organic Framework for High-efficiency Degradation of Methylene Blue in Water by Peroxymonosulfate Activation [J]. Acta Chimica Sinica, 2024, 82(2): 152-159. |
| [2] | Kai Zhang, Xiaojun Wu. Room-Temperature Ferromagnetism in Two-Dimensional Janus Titanium Chalcogenides★ [J]. Acta Chimica Sinica, 2023, 81(9): 1142-1147. |
| [3] | Zhengchu Zhang, Wei Xiong, Hua Lu. Preparation and Material Properties ofα-Helical Polypeptides Crosslinked Hydrogel★ [J]. Acta Chimica Sinica, 2023, 81(9): 1113-1119. |
| [4] | Xiao Wang, Xingwen Wang, Lehui Xiao. Nanocatalytic Mechanisms Investigated by Single Molecule Fluorescence Imaging at the Single-Particle Level [J]. Acta Chimica Sinica, 2023, 81(8): 1002-1014. |
| [5] | Rongjie Yang, Lin Zhou, Bin Su. Selective Detection of Vitamins A and C based on Covalent Organic Framework Modified Electrodes★ [J]. Acta Chimica Sinica, 2023, 81(8): 920-927. |
| [6] | Guoqing Cui, Yiyang Hu, Yingjie Lou, Mingxia Zhou, Yuming Li, Yajun Wang, Guiyuan Jiang, Chunming Xu. Research Progress on the Design, Preparation and Properties of Catalysts for CO2 Hydrogenation to Alcohols [J]. Acta Chimica Sinica, 2023, 81(8): 1081-1100. |
| [7] | Jia Liu, Guanghai Chen, Yiqun Chen, Jietao Jiang, Xiao Xiao, Qiang Wu, Lijun Yang, Xizhang Wang, Zheng Hu. Boosting the Supercapacitance Performance of Mesostructured Carbon Nanocages by Enlarging Pore Sizes via Carbothermal Reduction★ [J]. Acta Chimica Sinica, 2023, 81(7): 709-716. |
| [8] | Yuying Zhang, Xiao Cai, Weigang Hu, Guangjun Li, Yan Zhu. Pd and Hg Atoms Co-doped HgPdAu23(PET)18 Nanocluster★ [J]. Acta Chimica Sinica, 2023, 81(7): 703-708. |
| [9] | Xuefeng Liang, Jian Jing, Xin Feng, Yongze Zhao, Xinyuan Tang, Yan He, Lisheng Zhang, Huifang Li. Electronic Structure of Covalent Organic Frameworks COF66 and COF366: from Monomers to Two-Dimensional Framework [J]. Acta Chimica Sinica, 2023, 81(7): 717-724. |
| [10] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [11] | Congcong Ning, Qian Yang, Amin Mao, Zijia Tang, Yan Jin, Baoshan Hu. Research Progress in Controllable Preparation of Graphene Nanoribbons [J]. Acta Chimica Sinica, 2023, 81(4): 406-419. |
| [12] | Jinjing Liu, Na Yang, Li Li, Zidong Wei. Theoretical Study on the Regulation of Oxygen Reduction Mechanism by Modulating the Spatial Structure of Active Sites on Platinum★ [J]. Acta Chimica Sinica, 2023, 81(11): 1478-1485. |
| [13] | Dongxu Li, Xiang Xu, Jiage Song, Songting Liang, Yuang Fu, Xinhui Lu, Yingping Zou. Rotaxane Structure Optimizes the Photovoltaic Performance of Polymer Solar Cells★ [J]. Acta Chimica Sinica, 2023, 81(11): 1500-1507. |
| [14] | Zhengjia Zhao, Kang Liu, Yan Guo, Jipan Yu, Weiqun Shi. Research Progress of Transuranic Organometallic Chemistry [J]. Acta Chimica Sinica, 2023, 81(11): 1633-1641. |
| [15] | Xu Pu, Zejuan Li, Junqiu Shi, Yunqing Zhu, Jianzhong Du. Recent Advances in Organ-Targeting Polymeric Delivery Vectors for Nucleic Acids★ [J]. Acta Chimica Sinica, 2023, 81(10): 1438-1446. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||