Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (9): 1113-1119.DOI: 10.6023/A23050211 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Communication
投稿日期:2023-05-06
发布日期:2023-06-27
作者简介:基金资助:
Zhengchu Zhang, Wei Xiong( ), Hua Lu(
), Hua Lu( )
)
Received:2023-05-06
Published:2023-06-27
Contact:
*E-mail: About author:Supported by:Share
Zhengchu Zhang, Wei Xiong, Hua Lu. Preparation and Material Properties ofα-Helical Polypeptides Crosslinked Hydrogel★[J]. Acta Chimica Sinica, 2023, 81(9): 1113-1119.
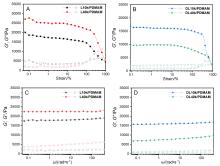

| 凝胶名称 | 储能模量G'/kPa | 耗散模量G"/kPa | 临界应变γ |
|---|---|---|---|
| L10k/PDMAM | 17.5 | 1.7 | 500% |
| L40k/PDMAM | 22.4 | 3.3 | 450% |
| DL10k/PDMAM | 16.0 | 2.3 | >1000% |
| DL40k/PDMAM | 7.8 | 0.8 | >1000% |
| 凝胶名称 | 储能模量G'/kPa | 耗散模量G"/kPa | 临界应变γ |
|---|---|---|---|
| L10k/PDMAM | 17.5 | 1.7 | 500% |
| L40k/PDMAM | 22.4 | 3.3 | 450% |
| DL10k/PDMAM | 16.0 | 2.3 | >1000% |
| DL40k/PDMAM | 7.8 | 0.8 | >1000% |

| 凝胶名称 | 弹性模量E/kPa | 断裂能/(kJ•m-3) | 断裂伸长率 |
|---|---|---|---|
| L10k/PDMAM | 34 | 22.4 | 320% |
| L40k/PDMAM | 32 | 43.6 | 350% |
| DL10k/PDMAM | 5.5 | 17.0 | 810% |
| DL40k/PDMAM | 7.1 | 17.2 | 650% |
| 凝胶名称 | 弹性模量E/kPa | 断裂能/(kJ•m-3) | 断裂伸长率 |
|---|---|---|---|
| L10k/PDMAM | 34 | 22.4 | 320% |
| L40k/PDMAM | 32 | 43.6 | 350% |
| DL10k/PDMAM | 5.5 | 17.0 | 810% |
| DL40k/PDMAM | 7.1 | 17.2 | 650% |
| [1] |
Ahmed, E. M. J. Adv. Res. 2015, 6, 105.
doi: 10.1016/j.jare.2013.07.006 pmid: 25750745 |
| [2] |
Zhang, Y. S.; Khademhosseini, A. Science 2017, 356, 3637.
|
| [3] |
Yu, H. C.; Zheng, S. Y.; Fang, L.; Ying, Z.; Du, M.; Wang, J.; Ren, K.; Wu, Z. L.; Zheng, Q. Adv. Mater. 2020, 32, 2005171.
doi: 10.1002/adma.v32.49 |
| [4] |
Han, Y.; Liu, C.; Xu, H.; Cao, Y. Chin. J. Chem. 2022, 40, 1578.
doi: 10.1002/cjoc.v40.13 |
| [5] |
Boehnke, N.; Cam, C.; Bat, E.; Segura, T.; Maynard, H. D. Biomacromolecules 2015, 16, 2101.
doi: 10.1021/acs.biomac.5b00519 |
| [6] |
Lee, K. Y.; Mooney, D. J. Chem. Rev. 2001, 101, 1869.
doi: 10.1021/cr000108x pmid: 11710233 |
| [7] |
Qiu, Y.; Park, K. Adv. Drug Deliv. Rev. 2001, 53, 321.
doi: 10.1016/S0169-409X(01)00203-4 |
| [8] |
Li, J.; Mooney, D. J. Nat. Rev. Mater. 2016, 1, 16071.
doi: 10.1038/natrevmats.2016.71 |
| [9] |
Wang, S. H.; Sun, L. N.; Cao, H.; Zhong, Y. M.; Shao, Z. Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese)
doi: 10.6023/A21050203 |
|
(王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.)
doi: 10.6023/A21050203 |
|
| [10] |
Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Chem. Rev. 2021, 121, 4309.
doi: 10.1021/acs.chemrev.0c01088 |
| [11] |
Taylor, D.; O’Mara, N.; Ryan, E.; Takaza, M.; Simms, C. Biomed. Mater. 2012, 6, 139.
|
| [12] |
Li, L.; Zhang, K.; Wang, T.; Wang, P.; Xue, B.; Cao, Y.; Zhu, L.; Jiang, Q. Mater. Des. 2020, 189, 108492.
doi: 10.1016/j.matdes.2020.108492 |
| [13] |
Wei, H.; Zhang, B.; Lei, M.; Lu, Z.; Liu, J.; Guo, B.; Yu, Y. ACS Nano 2022, 16, 4734.
doi: 10.1021/acsnano.1c11589 |
| [14] |
Cai, Y.; Shi, J.; Liu, F.; Li, H.; Man, X.; Guan, S. Chin. J. Chem. 2021, 39, 3085.
doi: 10.1002/cjoc.v39.11 |
| [15] |
Yang, Y. Y.; Wang, X.; Wu, D. C. Acta Chim. Sinica 2021, 79, 1. (in Chinese)
doi: 10.6023/A20080370 |
|
(杨艳宇, 王星, 吴德成, 化学学报, 2021, 79, 1.)
doi: 10.6023/A20080370 |
|
| [16] |
Fantner, G. E.; Oroudjev, E.; Schitter, G.; Golde, L. S.; Thurner, P.; Finch, M. M.; Turner, P.; Gutsmann, T.; Morse, D. E.; Hansma, H.; Hansma, P. K. Biophys. J. 2006, 90, 1411.
pmid: 16326907 |
| [17] |
Root, D. D.; Yadavalli, V. K.; Forbes, J. G.; Wang, K. Biophys. J. 2006, 90, 2852.
doi: 10.1529/biophysj.105.071597 |
| [18] |
Xu, Z.-H.; Li, X. Adv. Funct. Mater. 2011, 21, 3883.
doi: 10.1002/adfm.v21.20 |
| [19] |
Tian, Z.-Y.; Zhang, Z.; Wang, S.; Lu, H. Nat. Commun. 2021, 12, 1.
doi: 10.1038/s41467-020-20314-w |
| [20] |
Oelker, A. M.; Morey, S. M.; Griffith, L. G.; Hammond, P. T. Soft Matter 2012, 8, 10887.
doi: 10.1039/c2sm26487k |
| [21] |
Chen, C.; Lan, J.; Li, Y.; Liang, D.; Ni, X.; Liu, Q. Chem. Mater. 2019, 32, 1153.
doi: 10.1021/acs.chemmater.9b04160 |
| [22] |
Liu, R.; Wang, H.; Lu, W.; Cui, L.; Wang, S.; Wang, Y.; Chen, Q.; Guan, Y.; Zhang, Y. Chem. Eng. J. 2021, 415, 128839.
doi: 10.1016/j.cej.2021.128839 |
| [23] |
Liu, P.; Zhang, Y.; Guan, Y.; Zhang, Y. Adv. Mater. 2023, 2210021.
|
| [24] |
Liu, R.; Cui, L.; Wang, H.; Chen, Q.; Guan, Y.; Zhang, Y. ACS Appl. Mater. Interfaces 2021, 13, 42052.
doi: 10.1021/acsami.1c12687 |
| [25] |
Hou, Y.; Zhou, Y.; Wang, H.; Sun, J.; Wang, R.; Sheng, K.; Yuan, J.; Hu, Y.; Chao, Y.; Liu, Z.; Lu, H. ACS Cent. Sci. 2019, 5, 229.
doi: 10.1021/acscentsci.8b00548 |
| [26] |
Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubois, P.; Mason, A. F.; Hedrick, J. L.; Liao, Q.; Frank, C. W.; Kingsbury, K.; Hawker, C. J. Chem. Commun. 2006, 26, 2774.
|
| [27] |
Sakai, T.; Akagi, Y.; Kondo, S.; Chung, U. Soft Matter 2014, 10, 6658.
doi: 10.1039/C4SM00709C |
| [28] |
Nele, V.; Wojciechowski, J. P.; Armstrong, J. P.; Stevens, M. M. Adv. Funct. Mater. 2020, 30, 2002759.
doi: 10.1002/adfm.v30.42 |
| [29] |
Song, G.; Zhao, Z.; Peng, X.; He, C.; Weiss, R. A.; Wang, H. Macromolecules 2016, 49, 8265.
doi: 10.1021/acs.macromol.6b01448 |
| [30] |
Wilcox, K. G.; Dingle, M. E.; Saha, A.; Hore, M. J. A.; Morozova, S. Soft Matter 2022, 18, 6550.
doi: 10.1039/D2SM00921H |
| [31] |
Morton, L. D.; Hillsley, A.; Austin, M. J.; Rosales, A. M. J. Mater. Chem. B 2020, 8, 6925.
doi: 10.1039/d0tb00683a pmid: 32436556 |
| [32] |
Zhang, C.; Yuan, J.; Lu, J.; Hou, Y.; Xiong, W.; Lu, H. Biomaterials 2018, 178, 728.
doi: 10.1016/j.biomaterials.2018.01.052 |
| [1] | Yuqing Shi, Mingzhu Chu, Bo Han, Haojie Ma, Ran Li, Xueyan Hou, Yuqi Zhang, Ji-Jiang Wang. Smart Two-dimensional Photonic Crystal Hydrogel for Accurate Detection of Hg2+ [J]. Acta Chimica Sinica, 2024, 82(1): 9-15. |
| [2] | Hengjie Zhang, Kunrui Liu, Xianchun Chen, Zhipeng Gu, Yiwen Li. Design and Application of Light Responsive Smart Bio-adhesive Materials★ [J]. Acta Chimica Sinica, 2023, 81(12): 1739-1753. |
| [3] | Suhang Wang, Lingna Sun, Han Cao, Yiming Zhong, Zhengzhong Shao. Development of a Dual-drug-loaded Silk Fibroin Hydrogel and Study on Its Drugs Release Behaviors [J]. Acta Chimica Sinica, 2021, 79(8): 1023-1029. |
| [4] | Yanyu Yang, Xing Wang, Decheng Wu. Chitosan-Based High-Mechanical Double-Network Hydrogels: Construction, Modulation and Applications [J]. Acta Chimica Sinica, 2021, 79(1): 1-9. |
| [5] | Geng Huimin, Cui Jiwei, Hao Jingcheng. Mussel-Inspired Hydrogels for Tissue Healing [J]. Acta Chimica Sinica, 2020, 78(2): 105-113. |
| [6] | Zhang Yi, Chen Yong, Li Jingjing, Liang Lu, Liu Yu. Construction and Luminescent Behavior of Supramolecular Hydrogel with White-Light Emission [J]. Acta Chim. Sinica, 2018, 76(8): 622-626. |
| [7] | Shao Yu, Li Chuang, Zhou Xu, Chen Ping, Yang Zhongqiang, Li Zhibo, Liu Dongsheng. Responsive Polypeptide-DNA Hydrogel Crosslinked by G-quadruplex [J]. Acta Chim. Sinica, 2015, 73(8): 815-818. |
| [8] | Ren Kai, He Jinlin, Zhang Mingzu, Wu Yixian, Ni Peihong. Synthesis and Characterization of pH-Sensitive Copolymer mPEG-acetal-PIB and Fabrication of Hydrogel for Wound Dressing [J]. Acta Chim. Sinica, 2015, 73(10): 1038-1046. |
| [9] | Liu Shuilian, Zhou Yang, Chen Fuhua, Zhu Shoujin, Su Feng, Li Suming. Rheological Properties, Drug Release Behavior and Cytocompatibility of Novel Hydrogels Prepared from Carboxymethyl Chitosan [J]. Acta Chim. Sinica, 2015, 73(1): 47-52. |
| [10] | Liu Yanwei, Cao Hongyu, Tang Qian, Zheng Xuefang. Spectral Study on the Photoreduction of Cytochrome c under Macromolecular Crowding [J]. Acta Chimica Sinica, 2014, 72(2): 246-252. |
| [11] | Jin Sha, Pan Yuanjia, Wang Changchun. Reflux Precipitation Polymerization:A New Technology for Preparation of Monodisperse Polymer Nanohydrogels [J]. Acta Chimica Sinica, 2013, 71(11): 1500-1504. |
| [12] | Zhang Yaling, Yang Bin, Xu Liangxin, Zhang Xiaoyong, Tao Lei, Wei Yen. Self-healing Hydrogels Based on Dynamic Chemistry and Their Biomedical Applications [J]. Acta Chimica Sinica, 2013, 71(04): 485-492. |
| [13] | Song Qiusheng, Gao Kang, Yao Wei, Yang Yang, Ma Haihong. Thermosensitive Fluorescent Behavior and Mechanism of EuF3-NaYF4 Nanocrystals/PNIPAm Ternary Complex Hydrogel [J]. Acta Chimica Sinica, 2012, 70(20): 2155-2161. |
| [14] | Wang Zhen, Guo Dongsheng, Zhang Jie, Liu Yu. Electro-responsive Binary Hydrogels Based on Calixarene and Viologens [J]. Acta Chimica Sinica, 2012, 70(16): 1709-1715. |
| [15] | Liu Weitao, Liu Xuewen, Zhu Shen, Liu Xinyi, Han Guozhi. Ultrasonic Synthesis of pH-Sensitive PMAA Hydrogel [J]. Acta Chimica Sinica, 2012, 70(03): 272-276. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||