Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (12): 1502-1510.DOI: 10.6023/A21080385 Previous Articles Next Articles
Article
高霞a,*( ), 潘会宾b, 贺曾贤a, 杨柯a, 乔成芳a, 刘永亮a, 周春生a
), 潘会宾b, 贺曾贤a, 杨柯a, 乔成芳a, 刘永亮a, 周春生a
投稿日期:2021-08-16
发布日期:2021-10-08
通讯作者:
高霞
基金资助:
Xia Gaoa( ), Huibin Panb, Zengxian Hea, Ke Yanga, Chengfang Qiaoa, Yongliang Liua, Chunsheng Zhoua
), Huibin Panb, Zengxian Hea, Ke Yanga, Chengfang Qiaoa, Yongliang Liua, Chunsheng Zhoua
Received:2021-08-16
Published:2021-10-08
Contact:
Xia Gao
Supported by:Share
Xia Gao, Huibin Pan, Zengxian He, Ke Yang, Chengfang Qiao, Yongliang Liu, Chunsheng Zhou. Construction and Structure-Activity Relationship of Immobilized Enzyme Reactor Based on Al-MOF-Derived Al2O3 with Hierarchical Structure[J]. Acta Chimica Sinica, 2021, 79(12): 1502-1510.
| Enzyme | vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(L•mmol-1•s-1) |
|---|---|---|---|---|
| Free HRP | 0.5355 | 0.4093 | 21.4200 | 52.3333 |
| HRP@MHAl2O3 | 1.1254 | 0.1125 | 45.0160 | 400.1422 |
| HRP@PDA@MHAl2O3 | 1.3456 | 0.0807 | 53.8240 | 666.9641 |
| Enzyme | vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(L•mmol-1•s-1) |
|---|---|---|---|---|
| Free HRP | 0.5355 | 0.4093 | 21.4200 | 52.3333 |
| HRP@MHAl2O3 | 1.1254 | 0.1125 | 45.0160 | 400.1422 |
| HRP@PDA@MHAl2O3 | 1.3456 | 0.0807 | 53.8240 | 666.9641 |
| Enzyme | Ka×10-3/(L•mol-1) | ΔH/(kJ•mol-1) | ΔS/(J•mol-1•K-1) | n | TΔS/(kJ•mol-1) | ΔG/(kJ•mol-1) |
|---|---|---|---|---|---|---|
| Free HRP | 0.75±0.01 | 1088.0±0.01 | 3703.0±0.2 | 1.23±0.1 | 1104.0±0.1 | –16.41±0.04 |
| HRP@MHAl2O3 | 0.88±0.01 | 996.9±0.02 | 3409.0±0.1 | 1.36±0.1 | 1016.0±0.2 | –16.79±0.06 |
| HRP@PDA@MHAl2O3 | 1.03±0.01 | 142.2±0.01 | 534.4±0.1 | 6.91±0.1 | 159.3±0.2 | –17.19±0.06 |
| Enzyme | Ka×10-3/(L•mol-1) | ΔH/(kJ•mol-1) | ΔS/(J•mol-1•K-1) | n | TΔS/(kJ•mol-1) | ΔG/(kJ•mol-1) |
|---|---|---|---|---|---|---|
| Free HRP | 0.75±0.01 | 1088.0±0.01 | 3703.0±0.2 | 1.23±0.1 | 1104.0±0.1 | –16.41±0.04 |
| HRP@MHAl2O3 | 0.88±0.01 | 996.9±0.02 | 3409.0±0.1 | 1.36±0.1 | 1016.0±0.2 | –16.79±0.06 |
| HRP@PDA@MHAl2O3 | 1.03±0.01 | 142.2±0.01 | 534.4±0.1 | 6.91±0.1 | 159.3±0.2 | –17.19±0.06 |
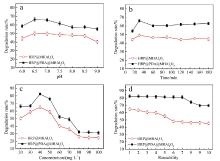
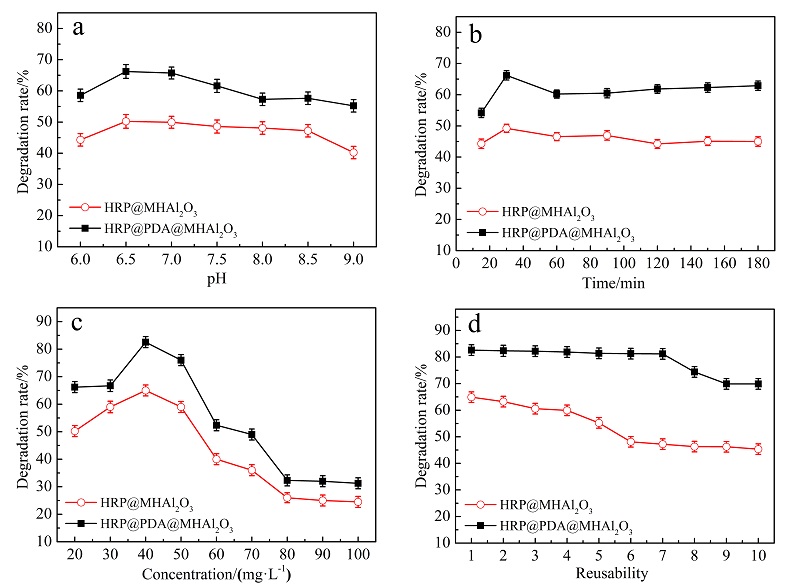
| [1] |
Zhou, Y. G.; Mohamadi, R. M.; Poudineh, M.; Kermanshah, L.; Ahmed, S.; Safaei, T. S.; Stojcic, J.; Nam, R. K.; Sargent, E. H.; Kelley, S. O. Small 2016, 12, 727.
doi: 10.1002/smll.v12.6 |
| [2] |
Chen, W.; Jin, B.; Hu, Y. L.; Lu, Y.; Xia, X. H. Small 2012, 8, 1001.
doi: 10.1002/smll.201102117 pmid: 22311804 |
| [3] |
Cui, J. W.; He, S.; Dai, S.; Liu, L. Y.; Zhao, A.; Lu, L.; Yang, P.; Chen, J.; Huang, N. Chem. Eng. J. 2021, 424, 130392.
doi: 10.1016/j.cej.2021.130392 |
| [4] |
Cheng, H. P.; Hu, M. C.; Zhai, Q. G.; Li, S. N.; Jiang, Y. C. Chem. Eng. J. 2018, 347, 703.
doi: 10.1016/j.cej.2018.04.083 |
| [5] |
Zhuang, W.; Quan, X. B.; Wang, Z. F.; Zhou, W. F.; Yang, P. P.; Ge, L.; Hernandez, B. V.; Wu, J. L.; Li, M.; Zhou, J.; Zhu, C. J.; Ying, H. J. Chem. Eng. J. 2020, 394, 125038.
|
| [6] |
Sankaran, R.; Show, P. L.; Chang, J. S. Biofuel. Bioprod. Bior. 2016, 10, 896.
doi: 10.1002/bbb.2016.10.issue-6 |
| [7] |
Zhao, X. B.; Qi, F.; Yuan, C. L.; Du, W.; Liu, D. H. Renew. Sust. Energy Rev. 2015, 44, 182.
|
| [8] |
An, K. J.; Kwon, S. G.; Park, M.; Na, H. B.; Baik, S.; Yu, J. H.; Kim, D.; Son, J. S.; Kim, Y.W.; Song, I. C.; Moon, W. K.; Park, H. M.; Hyeon, T. Nano Lett. 2008, 8, 4252.
doi: 10.1021/nl8019467 |
| [9] |
Xiong, S. L.; Zeng, H. C. Angew. Chem. Int. Ed. 2012, 51, 949.
doi: 10.1002/anie.v51.4 |
| [10] |
Chen, J.; He, S. M.; Huang, B.; Zhang, L. Y.; Qiao, Z. Q.; Wang, J.; Yang, G. C.; Huang, H.; Hao, Q. L. Appl. Surf. Sci. 2018, 457, 508.
doi: 10.1016/j.apsusc.2018.06.301 |
| [11] |
Journet, C.; Maser, W. K.; Bernier, P.; Loiseau, A.; Lamy de la Chapelle, M.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J. E. Nature 1997, 388, 756.
doi: 10.1038/41972 |
| [12] |
Ishaq, S.; Tamime, R.; Bilad, M. R.; Khan, A. L. Sep. Purif. Technol. 2019, 210, 442.
doi: 10.1016/j.seppur.2018.08.031 |
| [13] |
Deng, Q.; Wang, R. Catal. Commun. 2019, 120, 11.
doi: 10.1016/j.catcom.2018.11.009 |
| [14] |
Zhang, W. Q.; Li, Q. Y.; Yang, X. Y.; Ma, Z.; Wang, H. H.; Wang, X. J. Acta Chim. Sinica 2017, 75, 80 (in Chinese).
|
|
( 张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军, 化学学报, 2017, 75, 80.)
doi: 10.6023/A16090496 |
|
| [15] |
Small, L. J.; Hill, R. C.; Krumhansl, J. L.; Schindelholz, M. E.; Chen, Z.; Chapman, K. W.; Zhang, X.; Yang, S.; Schröder, M.; Nenoff, T. M. ACS Appl. Mater. Inter. 2019, 11, 27982.
doi: 10.1021/acsami.9b09938 |
| [16] |
Chang, Z.; Qiao, Y.; Yang, H. J.; Deng, H.; Zhu, X. Y.; He, P.; Zhou, H. S. Acta Chim. Sinica 2021, 79, 139 (in Chinese).
doi: 10.6023/A20090442 |
|
( 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎, 化学学报, 2021, 79, 139.)
doi: 10.6023/A20090442 |
|
| [17] |
Wu, M. X.; Yang, Y. W. Adv. Mater. 2017, 29, 1606134.
doi: 10.1002/adma.201606134 |
| [18] |
Chen, X. R.; Tong, R. L.; Shi, Z. Q.; Yang, B.; Liu, H.; Ding, S. P.; Wang, X.; Lei, Q. F.; Wu, J.; Fang, W. J. ACS. Appl. Mater. Inter. 2018, 10, 2328.
|
| [19] |
Liu, Y.; Shao, X. X.; Kong, D. Q.; Li, G. Q.; Li, Q. S. Colloids Surf. B Biointerfaces 2021, 197, 111450.
doi: 10.1016/j.colsurfb.2020.111450 |
| [20] |
Zhao, R. N.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Acta Chim. Sinica 2017, 75, 293 (in Chinese).
doi: 10.6023/A16110593 |
|
( 赵睿南, 胡满成, 李淑妮, 翟全国, 蒋育澄, 化学学报, 2017, 75, 293.)
doi: 10.6023/A16110593 |
|
| [21] |
Nadar, S.; Rathod, V. K. Int. J. Biol. Macromol. 2018, 120, 2293.
doi: 10.1016/j.ijbiomac.2018.08.126 |
| [22] |
Liu, X. P.; Yan, Z. Q.; Zhang, Y.; Liu, Z. W.; Sun, Y. H.; Ren, J. S.; Qu, X. G. ACS Nano 2019, 13, 5222.
doi: 10.1021/acsnano.8b09501 |
| [23] |
Yao, X. F.; Li, Y. W. Chin. Sci. Bull. 2015, 60, 1906 (in Chinese).
doi: 10.1360/N972015-00438 |
|
( 姚显芳, 李映伟, 科学通报, 2015, 60, 1906.)
|
|
| [24] |
Bhadra, B. N.; Vinu, A.; Serre, C.; Jhung, S. H. Mater. Today 2019, 25, 88.
doi: 10.1016/j.mattod.2018.10.016 |
| [25] |
Zhong, M.; Kong, L.; Li, N.; Liu, Y. Y.; Zhu, J.; Bu, X. H. Coord. Chem. Rev. 2019, 388, 172.
doi: 10.1016/j.ccr.2019.02.029 |
| [26] |
Salunkhe, R. R.; Kaneti, Y. V.; Yamauchi, Y. ACS Nano 2017, 11, 5293.
doi: 10.1021/acsnano.7b02796 pmid: 28613076 |
| [27] |
Wei, W. B.; Dong, S. Y.; Huang, G. Q.; Xie, Q.; Huang, T. L. Sens. Actuators B: Chem. 2018, 260, 189.
doi: 10.1016/j.snb.2017.12.207 |
| [28] |
Xie, W.; Zhou, L. J.; Xu, J.; Guo, Q. L.; Jiang, F. L.; Liu, Y. Acta Phys.-Chim. Sin. 2020, 36, 1905051 (in Chinese).
|
|
( 谢文, 周莲娇, 徐娟, 郭清莲, 蒋风雷, 刘义, 物理化学学报, 2020, 36, 1905051.)
|
|
| [29] |
Li, L.; Xiang, S. L.; Cao, S. Q.; Zhang, J. Y.; Ouyang, G. F.; Chen, L. P.; Su, C. Y. Nat. Commun. 2013, 4, 1774.
doi: 10.1038/ncomms2757 |
| [30] |
Chen, Y.; Xiao, Z.; Liu, Y.; Fan, L. Z. J. Mater. Chem. 2017, 5, 24178.
|
| [31] |
Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Song, J.; Jiang, Y. C. J. Chem. Technol. Biot. 2019, 94, 1249.
|
| [32] |
Song, Y. C.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Chem. J. Chin. Univ. 2019, 40, 1805 (in Chinese).
|
|
( 宋艺超, 胡满成, 李淑妮, 翟全国, 蒋育澄, 高等学校化学学报, 2019, 40, 1805.)
|
|
| [33] |
Yang, Y.; Wang, S.; Zhou, Z.; Zhang, R.; Shen, H.; Song, J.; Su, P.; Yang, Y. BioChem. Eng. J. 2018, 137, 108.
doi: 10.1016/j.bej.2018.05.019 |
| [34] |
Cao, D. L.; Cheng, W. J.; Tao, K.; Liang, Y. X. Macromol. Res. 2018, 26, 616.
doi: 10.1007/s13233-018-6087-z |
| [35] |
Xiang, L.; Xiao, T.; Mo, C. H.; Zhao, H. M.; Li, Y. W.; Li, H.; Cai, Q. Y.; Zhou, D. M.; Wong, M. H. Ecotoxicol. Environ. Saf. 2018, 154, 84.
doi: 10.1016/j.ecoenv.2018.01.032 |
| [36] |
Fu, P. F.; Ma, Y. H.; Lei, B. L.; Li, G.; Lin, X. F. Environ. Technol. 2021, 42, 659.
doi: 10.1080/09593330.2019.1642389 |
| [37] |
Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Jiang, Y. C. Catal. Sci. Technol. 2021, 11, 2446.
doi: 10.1039/D0CY02146F |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||