Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (10): 1376-1384.DOI: 10.6023/A22070288 Previous Articles Next Articles
Article
殷东a, 商宏怡a, 余文浩a,b, 向仕凯a, 胡平a, 赵可清a, 冯春a,*( ), 汪必琴a,*(
), 汪必琴a,*( )
)
投稿日期:2022-07-01
发布日期:2022-09-06
通讯作者:
冯春, 汪必琴
基金资助:
Dong Yina, Hongyi Shanga, Wenhao Yua,b, Shikai Xianga, Ping Hua, Keqing Zhaoa, Chun Fenga( ), Biqin Wanga(
), Biqin Wanga( )
)
Received:2022-07-01
Published:2022-09-06
Contact:
Chun Feng, Biqin Wang
Supported by:Share
Dong Yin, Hongyi Shang, Wenhao Yu, Shikai Xiang, Ping Hu, Keqing Zhao, Chun Feng, Biqin Wang. Synthesis, Mesomorphism and Gelation Properties of Triazole-Modified Triphenylene 2,3-Dicarboxylic Esters and 2,3-Dicarboxyimides[J]. Acta Chimica Sinica, 2022, 80(10): 1376-1384.
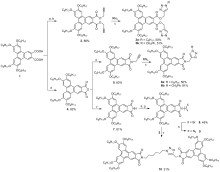

| Compd. | Mesophases, transition temperature and enthalpy changes | |
|---|---|---|
| 2nd heating/℃ (ΔH/(kJ•mol–1)) | 1st cooling/℃ (ΔH/(kJ•mol–1)) | |
| 2 | Cr 77 (45.8) Colh 142 (5.3) I | I 137 (5.0) Colh 5 (19.2) Cr |
| 5 | Cr 130 (55.5) Colh 224 (4.3) I | I 220 (4.9) Colh 84 (51.5) Cr |
| 3a | Cr 118 (33.6) I | I 101 (43.2) Cr |
| 3b | Cr 159 (41.6) I | I 123 (15.6) Cr |
| 6a | Cr 108 (36.6) Colh 215 (6.7) I | I 212 (5.6) Colh 63 (39.6) Cr |
| 6b | Cr 165 (31.2) Colh 223 (6.4) I | I 221 (7.4) Colh 88 (27.7) Cr |
| 10 | Cr 21 (2.4) Colh1 161 (1.6) Colh2187 (3.1) I | I 181 (4.3) Colh2 136 (1.2) Colh1 8 (2.4) Cr |
| Compd. | Mesophases, transition temperature and enthalpy changes | |
|---|---|---|
| 2nd heating/℃ (ΔH/(kJ•mol–1)) | 1st cooling/℃ (ΔH/(kJ•mol–1)) | |
| 2 | Cr 77 (45.8) Colh 142 (5.3) I | I 137 (5.0) Colh 5 (19.2) Cr |
| 5 | Cr 130 (55.5) Colh 224 (4.3) I | I 220 (4.9) Colh 84 (51.5) Cr |
| 3a | Cr 118 (33.6) I | I 101 (43.2) Cr |
| 3b | Cr 159 (41.6) I | I 123 (15.6) Cr |
| 6a | Cr 108 (36.6) Colh 215 (6.7) I | I 212 (5.6) Colh 63 (39.6) Cr |
| 6b | Cr 165 (31.2) Colh 223 (6.4) I | I 221 (7.4) Colh 88 (27.7) Cr |
| 10 | Cr 21 (2.4) Colh1 161 (1.6) Colh2187 (3.1) I | I 181 (4.3) Colh2 136 (1.2) Colh1 8 (2.4) Cr |
| Compd. | Temperature (Mesophase) | 2θ/(°) | dobs/nm | dcalcd/nm | I/% | hkl | Lattice parameters |
|---|---|---|---|---|---|---|---|
| 2 | 200 ℃ (Colh) | 4.87 19.73 25.13 | 1.81 0.45 0.35 | — | 100 0.1 0.1 | 100 hch hπ | a=2.09 nm A=3.78 nm2 Z=0.9 |
| 5 | 150 ℃ (Colh) | 4.89 19.76 25.83 | 1.81 0.45 0.35 | — | 100 0.1 22.1 | 100 hch hπ | a=2.08 nm |
| A=3.75 nm2 | |||||||
| Z=0.9 | |||||||
| 6a | 140 ℃ (Colh) | 4.28 19.94 25.34 | 2.06 0.44 0.35 | — | 100 0.1 18.8 | 100 hch hπ | a=2.38 nm A=4.91 nm2 Z=1.0 |
| 6b | 140 ℃ (Colh) | 4.46 19.73 25.6 | 1.98 0.45 0.35 | — | 100 1.0 10.5 | 100 hch hπ | a=2.29 nm A=4.54 nm2 Z=1.0 |
| 10 | 170 ℃ (Colh2) | 4.39 19.68 25.03 | 2.01 0.45 0.35 | — | 100 0.4 29.5 | 100 hch hπ | a=2.32 nm A=4.66 nm2 Z=0.5 |
| 60 ℃ (Colh1) | 4.48 10.12 12.66 15.52 20.95 23.13 25.66 | 1.97 0.87 0.70 0.57 0.42 0.38 0.35 | 1.97 0.85 0.68 0.57 — 0.38 — | 9.7 0.1 0.2 0.2 0.2 0.7 100 | 110 400 500 600 hch 730 hπ | a=3.94 nm A=13.444 nm2 Z=1.4 |
| Compd. | Temperature (Mesophase) | 2θ/(°) | dobs/nm | dcalcd/nm | I/% | hkl | Lattice parameters |
|---|---|---|---|---|---|---|---|
| 2 | 200 ℃ (Colh) | 4.87 19.73 25.13 | 1.81 0.45 0.35 | — | 100 0.1 0.1 | 100 hch hπ | a=2.09 nm A=3.78 nm2 Z=0.9 |
| 5 | 150 ℃ (Colh) | 4.89 19.76 25.83 | 1.81 0.45 0.35 | — | 100 0.1 22.1 | 100 hch hπ | a=2.08 nm |
| A=3.75 nm2 | |||||||
| Z=0.9 | |||||||
| 6a | 140 ℃ (Colh) | 4.28 19.94 25.34 | 2.06 0.44 0.35 | — | 100 0.1 18.8 | 100 hch hπ | a=2.38 nm A=4.91 nm2 Z=1.0 |
| 6b | 140 ℃ (Colh) | 4.46 19.73 25.6 | 1.98 0.45 0.35 | — | 100 1.0 10.5 | 100 hch hπ | a=2.29 nm A=4.54 nm2 Z=1.0 |
| 10 | 170 ℃ (Colh2) | 4.39 19.68 25.03 | 2.01 0.45 0.35 | — | 100 0.4 29.5 | 100 hch hπ | a=2.32 nm A=4.66 nm2 Z=0.5 |
| 60 ℃ (Colh1) | 4.48 10.12 12.66 15.52 20.95 23.13 25.66 | 1.97 0.87 0.70 0.57 0.42 0.38 0.35 | 1.97 0.85 0.68 0.57 — 0.38 — | 9.7 0.1 0.2 0.2 0.2 0.7 100 | 110 400 500 600 hch 730 hπ | a=3.94 nm A=13.444 nm2 Z=1.4 |


| Compd. | Solvent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Hex | DMF | THF | DCE | EA | Methanol | Acetonitrile | 1,4-Dioxane | |
| 3a | S | I | S | S | S | S | G (1) | G (2) | I |
| 3b | S | I | S | S | S | S | G (2) | G (1) | I |
| 6a | S | G (4) | G (4) | S | S | PS | I | I | PS |
| 6b | G (4) | G (2) | G (4) | S | S | G (4) | I | I | PS |
| 10 | S | PS | S | S | G (1) | PS | I | I | G (1) |
| Compd. | Solvent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Hex | DMF | THF | DCE | EA | Methanol | Acetonitrile | 1,4-Dioxane | |
| 3a | S | I | S | S | S | S | G (1) | G (2) | I |
| 3b | S | I | S | S | S | S | G (2) | G (1) | I |
| 6a | S | G (4) | G (4) | S | S | PS | I | I | PS |
| 6b | G (4) | G (2) | G (4) | S | S | G (4) | I | I | PS |
| 10 | S | PS | S | S | G (1) | PS | I | I | G (1) |


| [1] |
(a) Wohrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J. C.; Staffeld, P.; Baro, A.; Giesselmann, F.; Laschat, S. Chem. Rev. 2016, 116, 1139.
doi: 10.1021/acs.chemrev.5b00190 pmid: 11498585 |
|
(b) Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; Tosoni, M. Angew. Chem., Int. Ed. 2007, 46, 4832.
doi: 10.1002/anie.200604203 pmid: 11498585 |
|
|
(c) Kumar, S. Liq. Cryst. 2020, 47, 1195.
doi: 10.1080/02678292.2020.1720840 pmid: 11498585 |
|
|
(d) Pal, S. K.; Setia, S.; Avinash, B. S.; Kumar, S. Liq. Cryst. 2013, 40, 1769.
doi: 10.1080/02678292.2013.854418 pmid: 11498585 |
|
|
(e) Kumar, M.; Varshney, S.; Kumar, S. Polym. J. 2021, 53, 283.
pmid: 11498585 |
|
|
(f) Kumar, S. Liq. Cryst. 2004, 31, 1037.
doi: 10.1080/02678290410001724746 pmid: 11498585 |
|
|
(g) Schmidt-Mende, L.; Fechtenkotter, A.; Mullen, K.; Moons, E.; Friend, R. H.; MacKenzie, J. D. Science 2001, 293, 1119.
pmid: 11498585 |
|
|
(h) Zhu, X. M.; Bai, X. Y.; Wang, H. F.; Hu, P.; Wang, B. Q.; Zhao, K. Q. Acta Chim. Sinica 2021, 79, 1486. (in Chinese)
doi: 10.6023/A21080397 pmid: 11498585 |
|
|
(朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清, 化学学报, 2021, 79, 1486.)
doi: 10.6023/A21080397 pmid: 11498585 |
|
|
(i) Chen, S. S.; Li, T.; Zhao, D. H. Acta Phys. Chim. Sin. 2010, 26, 1124. (in Chinese)
doi: 10.3866/PKU.WHXB20100412 pmid: 11498585 |
|
|
(陈树森, 李田, 赵达慧, 物理化学学报, 2010, 26, 1124.)
pmid: 11498585 |
|
| [2] |
(a) Zhao, K. Q.; Wang, B. Q.; Hu, P.; Gao, C. Y.; Yuan, F. J.; Li, H. R. Chin. J. Chem. 2006, 24, 210.
doi: 10.1002/cjoc.200690040 |
|
(b) Zhao, K. Q.; Hu, P.; Wang, B. Q.; Yu, W. H.; Chen, H. M.; Wang, X. L.; Shimizu, Y. Chin. J. Chem. 2007, 25, 375.
doi: 10.1002/cjoc.200790072 |
|
| [3] |
Allen, M. T.; Diele, S.; Harris, K. D. M.; Hegmann, T.; Kariuki, B. M.; Lose, D.; Preece, J. A.; Tschierske, C. J. Mater. Chem. 2001, 11, 302.
doi: 10.1039/b006916g |
| [4] |
Cammidge, A. N.; Chausson, C.; Gopee, H.; Li, J.; Hughes, D. L. Chem. Commun. 2009, 7375.
|
| [5] |
(a) Lavigueur, C.; Foster, E. J.; Williams, V. E. J. Am. Chem. Soc. 2008, 130, 11791.
doi: 10.1021/ja803406k |
|
(b) Voisin, E.; Johan Foster, E.; Rakotomalala, M.; Williams, V. E. Chem. Mater. 2009, 21, 3251.
|
|
| [6] |
Osawa, T.; Kajitani, T.; Hashizume, D.; Ohsumi, H.; Sasaki, S.; Takata, M.; Koizumi, Y.; Saeki, A.; Seki, S.; Fukushima, T. Angew. Chem., Int. Ed. 2012, 51, 7990.
doi: 10.1002/anie.201203077 |
| [7] |
Gao, X.; Hu, Y. J. Mater. Chem. C 2014, 2, 3099.
doi: 10.1039/C3TC32046D |
| [8] |
Gudeika, D. Synth. Met. 2020, 262, 116328.
doi: 10.1016/j.synthmet.2020.116328 |
| [9] |
(a) Wurthner, F.; Saha-Moller, C. R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Chem. Rev. 2016, 116, 962.
doi: 10.1021/acs.chemrev.5b00188 |
|
(b) Hu, Y. H.; Wu, W. L.; Yu, L. Y.; Luo, K. J.; Xu, X. P.; Li, Y.; Peng, Q. Acta Chim. Sinica 2020, 78, 1246. (in Chinese)
doi: 10.6023/A20070282 |
|
|
(胡瑜辉, 武文林, 于立扬, 骆开均, 徐小鹏, 李瑛, 彭强, 化学学报, 2020, 78, 1246.)
doi: 10.6023/A20070282 |
|
| [10] |
Pu, J.; Yang, T.; Wang, Y.; Zhu, Q.; Yang, M.; Liu, C.; Wang, W. Liq. Cryst. 2020, 47, 291.
doi: 10.1080/02678292.2019.1645899 |
| [11] |
(a) Psutka, K. M.; Bozek, K. J. A.; Maly, K. E. Org. Lett. 2014, 16, 5442.
doi: 10.1021/ol502678m pmid: 31362501 |
|
(b) Psutka, K. M.; LeDrew, J.; Taing, H.; Eichhorn, S. H.; Maly, K. E. J. Org. Chem. 2019, 84, 10796.
doi: 10.1021/acs.joc.9b01330 pmid: 31362501 |
|
| [12] |
Yin, J.; Qu, H. M.; Zhang, K.; Luo, J.; Zhang, X. J.; Chi, C. Y.; Wu, J. S. Org. Lett. 2009, 11, 3028.
doi: 10.1021/ol901041n |
| [13] |
(a) Foster, E. J.; Jones, R. B.; Lavigueur, C.; Williams, V. E. J. Am. Chem. Soc. 2006, 128, 8569.
pmid: 16802823 |
|
(b) Boden, N.; Bushby, R. J.; Lu, Z. B.; Cammidge, A. N. Liq. Cryst. 1999, 26, 495.
doi: 10.1080/026782999204930 pmid: 16802823 |
|
| [14] |
Feng, C.; Tian, X. L.; Zhou, J.; Xiang, S. K.; Yu, W. H.; Wang, B. Q.; Hu, P.; Redshaw, C.; Zhao, K. Q. Org. Biomol. Chem. 2014, 12, 6977.
doi: 10.1039/C4OB01503G |
| [15] |
(a) Han, X. D.; Tian, X. L.; Yu, W. H.; Xiang, S. K.; Hu, P.; Wang, B. Q.; Zhao, K. Q.; Chen, X. Z.; Feng, C. Liq. Cryst. 2017, 44, 1727.
doi: 10.1080/02678292.2017.1329557 |
|
(b) Feng, C.; Ding, Y. H.; Han, X. D.; Yu, W. H.; Xiang, S. K.; Wang, B. Q.; Hu, P.; Li, L. C.; Chen, X. Z.; Zhao, K. Q. Dyes Pigm. 2017, 139, 87.
doi: 10.1016/j.dyepig.2016.12.001 |
|
|
(c) Fan, S. Y.; Xu, H. T.; Li, Q. G.; Fang, D. M.; Yu, W. H.; Xiang, S. K.; Hu, P.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Liq. Cryst. 2019, 47, 1041.
doi: 10.1080/02678292.2019.1704898 |
|
|
(d) Du, Y.; Zhao, C. L.; Fan, S. Y.; Yu, W. H.; Xiang, S. K.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Liq. Cryst. 2022, 49, 719.
doi: 10.1080/02678292.2021.2006811 |
|
|
(e) Xia, X.; Zhao, C. L.; Xu, H. T.; Yu, W. H.; Xiang, S. K.; Li, L. C.; Zhao, K. Q.; Feng, C.; Wang, B. Q. Dyes Pigm. 2022, 199, 110114.
doi: 10.1016/j.dyepig.2022.110114 |
|
| [16] |
Berg, R.; Straub, B. F. Beilstein J. Org. Chem. 2013, 9, 2715.
doi: 10.3762/bjoc.9.308 |
| [17] |
(a) Rodrigues, L. D.; Sunil, D.; Chaithra, D.; Bhagavath, P. J. Mol. Liq. 2020, 297, 111909.
doi: 10.1016/j.molliq.2019.111909 |
|
(b) Liu, Q. S.; Lü, Y. F.; Bao, P. L.; Yue, H. L.; Wei, W. Chin. J. Org. Chem. 2020, 40, 4015. (in Chinese)
doi: 10.6023/cjoc202008042 |
|
|
(刘启顺, 吕玉芬, 鲍鹏丽, 岳会兰, 魏伟, 有机化学, 2020, 40, 4015.)
doi: 10.6023/cjoc202008042 |
|
|
(c) Zhang, H. L.; Cheng, J. J.; Zou, G. J. Funct. Polym. 2019, 32, 728. (in Chinese)
|
|
|
(张红莉, 程军杰, 邹纲, 功能高分子学报, 2019, 32, 728.)
|
|
|
(d) Cheng, H. F. Ph.D. Dissertation, Yunnan University, Kunming, 2018. (in Chinese)
|
|
|
((程慧芳, 博士论文, 云南大学, 昆明, 2018.)
|
|
| [18] |
Gallardo, H.; Westphal, E. Curr. Org. Synth. 2015, 12, 806.
doi: 10.2174/157017941206150828113416 |
| [19] |
(a) Cheng, H. M.; Ma, C.; Chen, Y.; Ni, H. L.; Feng, C.; Wang, B. Q.; Zhao, K. Q.; Yu, W. H.; Hu, P. Liq. Cryst. 2017, 44, 1450.
doi: 10.1080/02678292.2017.1282047 |
|
(b) Xia, M. H.; Chen, Y.; Chen, Z. R.; Yu, W. H.; Cheng, H. M.; Feng, C.; Ni, H. L.; Wang, B. Q.; Zhao, K. Q.; Hu, P. Liq. Cryst. 2022, 49, 72.
doi: 10.1080/02678292.2021.1944358 |
|
|
(c) Xiong, D.; Xiong, X. P.; Chen, Z. R.; Yu, W. H.; Feng, C.; Chen, H. M.; Ni, H. L.; Wang, B. Q.; Zhao, K. Q.; Hu, P. Liq. Cryst. 2022, 49, 1261.
doi: 10.1080/02678292.2022.2066731 |
|
| [20] |
Benbayer, C.; Kheddam, N.; Saïdi-Besbes, S.; de Givenchy, E. T.; Guittard, F.; Grelet, E.; Safer, A. M.; Derdour, A. J. Mol. Struct. 2013, 1034, 22.
doi: 10.1016/j.molstruc.2012.09.020 |
| [21] |
Park, S.; Cho, B. K. Soft Matter 2015, 11, 94.
doi: 10.1039/C4SM02004A |
| [22] |
Bhalla, V.; Singh, H.; Kumar, M.; Prasad, S. K. Langmuir 2011, 27, 15275.
doi: 10.1021/la203774p |
| [23] |
(a) Yu, W. H.; Nie, S. C.; Bai, Y. F.; Jing, Y.; Wang, B. Q.; Hu, P.; Zhao, K. Q. Sci. China: Chem. 2010, 53, 1134.
doi: 10.1007/s11430-010-4010-3 |
|
(b) Neier, R.; Thevenet, D. Synthesis 2011, 2011, 3801.
doi: 10.1055/s-0031-1289573 |
|
| [24] |
Zhao, K. Q.; Bai, Y. F.; Hu, P.; Wang, B. Q.; Shimizu, Y. Mol. Cryst. Liq. Cryst. 2009, 509, 819.
|
| [25] |
Godbert, N.; Crispini, A.; Ghedini, M.; Carini, M.; Chiaravalloti, F.; Ferrise, A. J. Appl. Cryst. 2014, 47, 668.
doi: 10.1107/S1600576714003240 |
| [26] |
Yang, Y.; Wang, H.; Wang, H. F.; Liu, C. X.; Zhao, K. Q.; Wang, B. Q.; Hu, P.; Monobe, H.; Heinrich, B.; Donnio, B. Cryst. Growth Des. 2018, 18, 4296.
doi: 10.1021/acs.cgd.8b00083 |
| [1] | Liwei Hu, Xianhu Liu, Chuntai Liu, Yanlin Song, Mingzhu Li. Self-assembly Fabrication and Applications of Photonic Crystal Structure Color Materials★ [J]. Acta Chimica Sinica, 2023, 81(7): 809-819. |
| [2] | Lanying Li, Qing Tao, Yanli Wen, Lele Wang, Ruiyan Guo, Gang Liu, Xiaolei Zuo. Poly-adenine-based DNA Probes and Their Applications in Biosensors★ [J]. Acta Chimica Sinica, 2023, 81(6): 681-690. |
| [3] | Zeng Chongyang, Hu Ping, Wang Biqin, Fang Wenyan, Zhao Keqing, Donnio Bertrand. Star-shaped Triphenylene-triazine Multi-stimuli Responsive Discotic Liquid Crystals: Synthesis, Properties and Applications [J]. Acta Chimica Sinica, 2023, 81(5): 469-479. |
| [4] | Xu Pu, Zejuan Li, Junqiu Shi, Yunqing Zhu, Jianzhong Du. Recent Advances in Organ-Targeting Polymeric Delivery Vectors for Nucleic Acids★ [J]. Acta Chimica Sinica, 2023, 81(10): 1438-1446. |
| [5] | Jamshid Kadirkhanov, Feng Zhong, Wenjian Zhang, Chunyan Hong. Preparation of Multi-chambered Vesicles by Polymerization-induced Self-assembly and the Influence of Solvophilic Fragments in the Core-forming Blocks [J]. Acta Chimica Sinica, 2022, 80(7): 913-920. |
| [6] | Zhiqin Wang, Bo Xiang, Xiaoyu Huang, Guolin Lu. Effect of Phosphotungstic Acid on Self-seeding of Oligo(p-phenylenevinylene)-b-poly(2-vinylpyridine)※ [J]. Acta Chimica Sinica, 2022, 80(3): 297-302. |
| [7] | Yanmei Jin, Ye Meng, Yuan Li, Jianhua Shi, Lei Deng. Supramolecular Self-assembly of Symmetric Dicyclohexanocucurbit[6]uril and Nicotinic Hydrazide [J]. Acta Chimica Sinica, 2022, 80(1): 44-48. |
| [8] | Xusheng Wang, Xu Yang, Chunhui Chen, Hongfang Li, Yuanbiao Huang, Rong Cao. Graphene Quantum Dots Supported on Fe-based Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction※ [J]. Acta Chimica Sinica, 2022, 80(1): 22-28. |
| [9] | Weihua Li. “Bridge” Makes Differences to the Self-assembly of Block Copolymers [J]. Acta Chimica Sinica, 2021, 79(2): 133-138. |
| [10] | Xue-Min Zhu, Xiao-Yan Bai, Hai-Feng Wang, Ping Hu, Bi-Qin Wang, Ke-Qing Zhao. Benzo[g]chrysene Discotic Liquid Crystals: Synthesis, Columnar Mesophase and Photophysical Properties [J]. Acta Chimica Sinica, 2021, 79(12): 1486-1493. |
| [11] | Zhao Jingjing, Zhang Zhengzhong, Chen Xiaolang, Wang Bei, Deng Jinyuan, Zhang Dieqing, Li Hexing. Microwave-induced Assembly of CuS@MoS2 Core-shell Nanotubes and Study on Their Photocatalytic Fenton-like Reactions [J]. Acta Chimica Sinica, 2020, 78(9): 961-967. |
| [12] | Jiang Jinhui, Zhu Yunqing, Du Jianzhong. Challenges and Perspective on Ring-Opening Polymerization-Induced Self-Assembly [J]. Acta Chimica Sinica, 2020, 78(8): 719-724. |
| [13] | Yin Cen, Wang Zikuan, Liu Dan, Peng Zhantao, Song Huanjun, Zhu Hao, Chen Qiwei, Wu Kai. Adsorption and Self-assembly of meso-tetra(p-methoxyphenyl)-porphyrinatocobalt(II) on Coinage Metal Surfaces [J]. Acta Chimica Sinica, 2020, 78(7): 695-702. |
| [14] | Liu Mingqian, Wan Xizi, Wang Shutao. Super Adhesive of Nanoparticle Solutions [J]. Acta Chimica Sinica, 2020, 78(6): 463-465. |
| [15] | Qie Shuyan, Hao Ying, Liu Zongjian, Wang Jin, Xi Jianing. Advances in Cyclodextrin Polymers and Their Applications in Biomedicine [J]. Acta Chimica Sinica, 2020, 78(3): 232-244. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||