Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (10): 1369-1375.DOI: 10.6023/A22080348 Previous Articles Next Articles
Communication
徐云芳a, 李阳a, 付梓桐a, 林绍艳b, 祝洁a, 吴磊a,*( )
)
投稿日期:2022-08-07
发布日期:2022-09-29
通讯作者:
吴磊
基金资助:
Yunfang Xua, Yang Lia, Zitong Fua, Shaoyan Linb, Jie Zhua, Lei Wua( )
)
Received:2022-08-07
Published:2022-09-29
Contact:
Lei Wu
Supported by:Share
Yunfang Xu, Yang Li, Zitong Fu, Shaoyan Lin, Jie Zhu, Lei Wu. Palladium-catalyzed Stereoselective Synthesis of (Z)-[3]Dendralenes[J]. Acta Chimica Sinica, 2022, 80(10): 1369-1375.
| Entry | Catalyst | Solvent | Time/h | Base | Yieldb/% | |
|---|---|---|---|---|---|---|
| 3a (Z/E)c | 4a | |||||
| 1 | Pd(PPh3)2Cl2 | 1,4-dioxane | 18 | K2CO3 | 24 (2:1) | 0 |
| 2 | Pd(PPh3)2Cl2 | DMF | 18 | K2CO3 | 30 (2.3:1) | 0 |
| 3 | Pd(PPh3)2Cl2 | EA | 18 | K2CO3 | trace | 0 |
| 4 | Pd(PPh3)2Cl2 | MeOH | 18 | K2CO3 | 50 (4:1) | 0 |
| 5 | Pd(PPh3)2Cl2 | MeOH | 9 | K2CO3 | 56 (4.3:1) | 0 |
| 6 | Pd(PPh3)2Cl2 | MeOH | 2 | K2CO3 | 67 (5:1) | 0 |
| 7 | Pd(PPh3)2Cl2 | MeOH | 0.5 | K2CO3 | 23 (Z) | 70 |
| 8 | Pd(OAc)2 | MeOH | 2 | K2CO3 | trace | 0 |
| 9 | Pd(PPh3)4 | MeOH | 2 | K2CO3 | 57 (4:1) | 0 |
| 10 | Pd(PhCN)Cl2 | MeOH | 2 | K2CO3 | 32 (4.2:1) | 0 |
| 11 | Rh(PPh3)2Cl | MeOH | 2 | K2CO3 | NDd | 0 |
| 12 | Pd(PPh3)2Cl2 | MeOH | 2 | DBU | 67 (5.3:1) | 0 |
| 13 | Pd(PPh3)2Cl2 | MeOH | 2 | DMAP | 34 (5.2:1) | 0 |
| 14 | Pd(PPh3)2Cl2 | MeOH | 2 | DIPEA | 40 (5:1) | 0 |
| 15 | Pd(PPh3)2Cl2 | MeOH | 2 | Cs2CO3 | 37 (3.2:1) | 0 |
| 16 | Pd(PPh3)2Cl2 | MeOH | 2 | KHCO3 | 46 (5:1) | 0 |
| Entry | Catalyst | Solvent | Time/h | Base | Yieldb/% | |
|---|---|---|---|---|---|---|
| 3a (Z/E)c | 4a | |||||
| 1 | Pd(PPh3)2Cl2 | 1,4-dioxane | 18 | K2CO3 | 24 (2:1) | 0 |
| 2 | Pd(PPh3)2Cl2 | DMF | 18 | K2CO3 | 30 (2.3:1) | 0 |
| 3 | Pd(PPh3)2Cl2 | EA | 18 | K2CO3 | trace | 0 |
| 4 | Pd(PPh3)2Cl2 | MeOH | 18 | K2CO3 | 50 (4:1) | 0 |
| 5 | Pd(PPh3)2Cl2 | MeOH | 9 | K2CO3 | 56 (4.3:1) | 0 |
| 6 | Pd(PPh3)2Cl2 | MeOH | 2 | K2CO3 | 67 (5:1) | 0 |
| 7 | Pd(PPh3)2Cl2 | MeOH | 0.5 | K2CO3 | 23 (Z) | 70 |
| 8 | Pd(OAc)2 | MeOH | 2 | K2CO3 | trace | 0 |
| 9 | Pd(PPh3)4 | MeOH | 2 | K2CO3 | 57 (4:1) | 0 |
| 10 | Pd(PhCN)Cl2 | MeOH | 2 | K2CO3 | 32 (4.2:1) | 0 |
| 11 | Rh(PPh3)2Cl | MeOH | 2 | K2CO3 | NDd | 0 |
| 12 | Pd(PPh3)2Cl2 | MeOH | 2 | DBU | 67 (5.3:1) | 0 |
| 13 | Pd(PPh3)2Cl2 | MeOH | 2 | DMAP | 34 (5.2:1) | 0 |
| 14 | Pd(PPh3)2Cl2 | MeOH | 2 | DIPEA | 40 (5:1) | 0 |
| 15 | Pd(PPh3)2Cl2 | MeOH | 2 | Cs2CO3 | 37 (3.2:1) | 0 |
| 16 | Pd(PPh3)2Cl2 | MeOH | 2 | KHCO3 | 46 (5:1) | 0 |


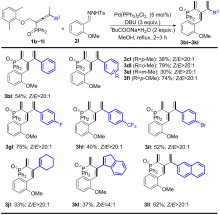
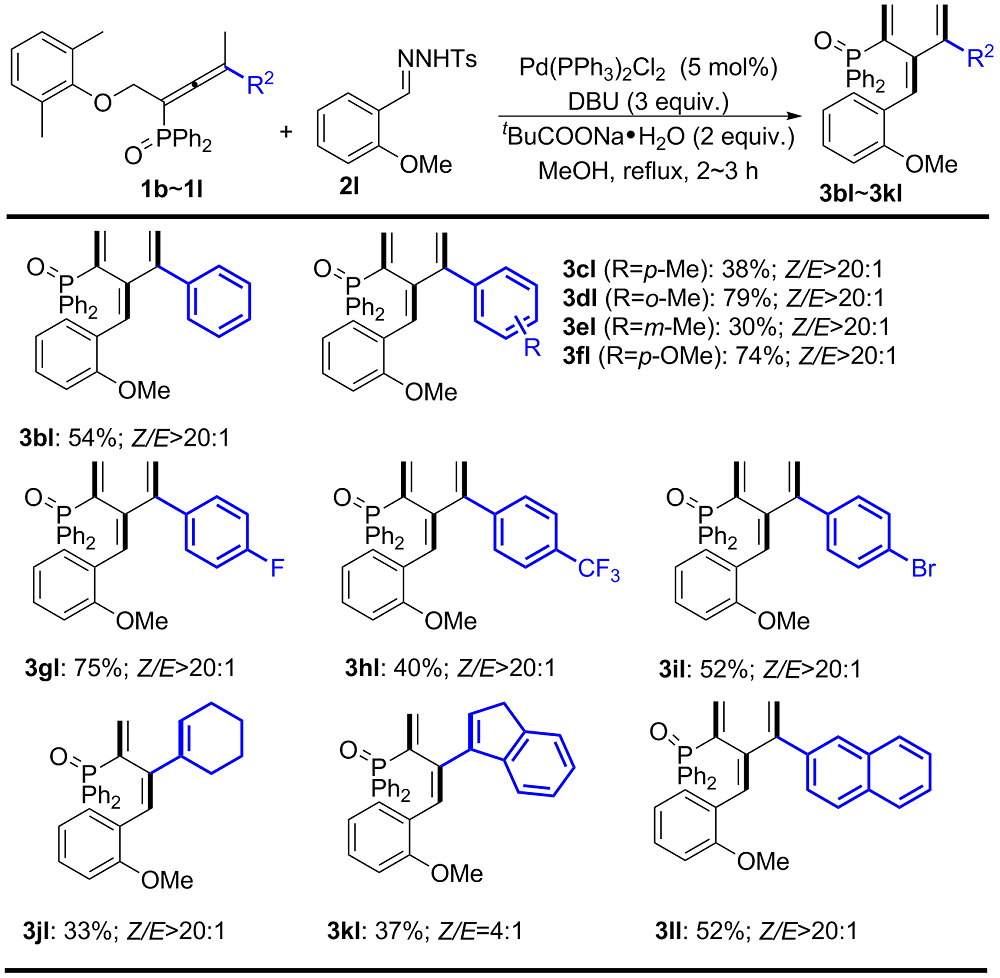
| [1] |
(a) Hopf, H. Angew. Chem., Int. Ed. 1984, 23, 948.
|
|
(b) Hopf, H. Nature 2009, 460, 183.
doi: 10.1038/460183a |
|
|
(c) Hopf, H. Angew. Chem., Int. Ed. 2001, 40, 705.
doi: 10.1002/1521-3773(20010216)40:4【-逻*辑*与-】#x00026;lt;705::AID-ANIE7050【-逻*辑*与-】#x00026;gt;3.0.CO;2-8 |
|
|
(d) Sherburn, M. S. Acc. Chem. Res. 2015, 48, 1961.
doi: 10.1021/acs.accounts.5b00242 |
|
|
(e) Hopf, H.; Sherburn, M. S. Cross Conjugation-Modern Dendralene, Radialene and Fulvene Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016.
|
|
| [2] |
Zhu, J.; Yang, W.-C.; Zhang, C.-Y. Chin. J. Org. Chem. 2020, 40, 1081. (in Chinese)
|
|
(祝洁, 杨文超, 张乘运, 有机化学, 2020, 40, 1081.)
|
|
| [3] |
Hopf, H.; Sherburn, M. S. Angew. Chem., Int. Ed. 2012, 51, 2302.
|
| [4] |
(a) Tattje, D. H. E.; Bos, R.; Bruins, A. P. Planta Med. 1980, 38, 79.
doi: 10.1055/s-2008-1074841 |
|
(b) Radovic, B. S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Food Chem. 2001, 72, 511.
doi: 10.1016/S0308-8146(00)00263-6 |
|
|
(c) Sefton, M. A.; Francis, I. L.; Williams, P. J. Food Sci. 1994, 59, 142.
doi: 10.1111/j.1365-2621.1994.tb06919.x |
|
| [5] |
Tsuge, O.; Wada, E.; Kanemasa, S. Chem. Lett. 1983, 12, 1525.
doi: 10.1246/cl.1983.1525 |
| [6] |
(a) Bonnett, R.; Davies, J. E.; Hursthouse, M. B. Nature 1976, 262, 326.
doi: 10.1038/262326a0 pmid: 17386601 |
|
(b) Tattje, D. H. E.; Bos, R.; Bruins, A. P. Planta Med. 1980, 38, 79.
doi: 10.1055/s-2008-1074841 pmid: 17386601 |
|
|
(c) Sefton, M. A.; Francis, I. L.; Williams, P. J. J. Food Sci. 1994, 59, 142.
doi: 10.1111/j.1365-2621.1994.tb06919.x pmid: 17386601 |
|
|
(d) Echard, J. P.; Benoit, C.; Peris-Vicente, J.; Malecki, V.; Gimeno-Adelantado, J. V.; Vaiedelich, S. Anal. Chim. Acta 2007, 584, 172.
pmid: 17386601 |
|
| [7] |
(a) Toombs-Ruane, H.; Pearson, E. L.; Paddon-Row, M. N.; Sherburn, M. S. Chem. Commun. 2012, 48, 6640.
|
|
(b) Deng, Y.; Bartholomeyzik, T.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2013, 52, 6283.
doi: 10.1002/anie.201301167 |
|
|
(c) Volla, C. M. R.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2013, 52, 14209.
doi: 10.1002/anie.201308448 |
|
|
(d) Takagi, T.; Toda, T.; Miya, M.; Takenaka, K. Macromolecules 2021, 54, 4327.
|
|
|
(e) Qiu, Y.; Posevins, D.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2017, 56, 13112.
doi: 10.1002/anie.201706211 |
|
|
(f) Xu, L.-G.; Wang, Z.-X. Adv. Synth. Catal. 2022, 364, 2753.
doi: 10.1002/adsc.202200516 |
|
| [8] |
Wang, H.; Beiring, B.; Yu, D.-G.; Collins, K. D.; Glorius, F. Angew. Chem., Int. Ed. 2013, 52, 12430.
doi: 10.1002/anie.201306754 |
| [9] |
Xia, Y.-T.; Xie, X.-Y.; Cui, S.-H.; Ji, Y.-G.; Wu, L. Chem. Commun. 2019, 55, 11699.
doi: 10.1039/C9CC05928H |
| [10] |
Xia, Y.-T.; Wu, J.-J.; Zhang, C.-Y.; Mao, M.; Ji, Y.-G.; Wu, L. Org. Lett. 2019, 21, 6383.
doi: 10.1021/acs.orglett.9b02287 |
| [11] |
George, J.; Ward, J. S.; Sherburn, M. S. Chem. Sci. 2019, 10, 9969.
doi: 10.1039/C9SC03976G |
| [12] |
Mao, M.; Zhang, L.; Chen, Y.-Z.; Zhu, J.; Wu, L. ACS Catal. 2017, 7, 181.
doi: 10.1021/acscatal.6b02972 |
| [13] |
Chang, S.; Grubbs, R. H. J. Org. Chem. 1998, 63, 864.
doi: 10.1021/jo9712198 |
| [14] |
Costa, M.; Dias, T. A.; Brito, A.; Proenca, F. Eur. J. Med. Chem. 2016, 123, 487.
doi: 10.1016/j.ejmech.2016.07.057 |
| [15] |
(a) Oliveira-Pinto, S.; Pontes, O.; Baltazar, F.; Costa, M. Eur. J. Pharm. 2022, 887, 173452.
doi: 10.1016/j.ejphar.2020.173452 |
|
(b) Patil, S. A.; Patil, R.; Pfeffer, L. M.; Miller, D. D. Future Med. Chem. 2013, 5, 1647.
doi: 10.4155/fmc.13.126 |
|
| [16] |
(a) Xiao, Q.; Zhang, Y.; Wang, J.-B. Acc. Chem. Res. 2013, 46, 236.
doi: 10.1021/ar300101k |
|
(b) Xia, Y.; Qiu, D.; Wang, J.-B. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
|
|
(c) Xia, Y.; Wang, J.-B. J. Am. Chem. Soc. 2020, 142, 10592.
doi: 10.1021/jacs.0c04445 |
|
|
(d) Li, S.-C.; Hou, B.; Wang, J.-B. J. Org. Chem. 2021, 86, 5371.
doi: 10.1021/acs.joc.0c03033 |
|
|
(e) José, B.; María, T.-G.; Fernando, A.; Carlos, V. Adv. Synth. Catal. 2010, 352, 3235.
doi: 10.1002/adsc.201000700 |
|
| [17] |
Chen, Y.-Z.; Zhang, L.; Lu, A.-M.; Yang, F.; Wu, L. J. Org. Chem. 2015, 80, 673.
doi: 10.1021/jo502485u |
| [18] |
Zhou, Z.; Liu, Y.; Chen, J.-F.; Yao, E.; Cheng, J. Org. Lett. 2016, 18, 5268.
doi: 10.1021/acs.orglett.6b02583 |
| [1] | Yaning Li, Xiaoyan Wang, Yong Tang. The Regulation of Stereoselectivity in Radical Polymerization★ [J]. Acta Chimica Sinica, 2024, 82(2): 213-225. |
| [2] | Dawei Zhang, Haiyang Zhao, Xiaotian Feng, Yucheng Gu, Xingang Zhang. Palladium-Catalyzed Cross-Coupling of Heteroaryl Bromides with gem-Difluoroallylborons [J]. Acta Chimica Sinica, 2024, 82(2): 105-109. |
| [3] | Yixiu Ge, Zaozao Qiu, Zuowei Xie. Pd-Catalyzed One-Pot Synthesis of Difunctionalized o-Carboranes via Construction of B—C and B—Heteroatom Bonds※ [J]. Acta Chimica Sinica, 2022, 80(4): 432-437. |
| [4] | Li Zhong-Yuan, Jing Kun, Li Qi-Li, Wang Guan-Wu. Palladium-Catalyzed Decarboxylative Coupling of Potassium Oxalate Monoester with 2-Aryloxypyridines [J]. Acta Chim. Sinica, 2019, 77(8): 729-734. |
| [5] | Li Shu-Sen, Wang Jianbo. Recent Advance in Asymmetric Trifluoromethylthiolation [J]. Acta Chim. Sinica, 2018, 76(12): 913-924. |
| [6] | Zhou Xiao-Le, Su Yong-Liang, Wang Pu-Sheng, Gong Liu-Zhu. Asymmetric Allylic C-H Alkylation of 1,4-Dienes with Aldehydes [J]. Acta Chim. Sinica, 2018, 76(11): 857-861. |
| [7] | Zhang Zi-Jing, Tao Zhong-Lin, Arafate Adele, Gong Liu-Zhu. Asymmetric Carbonyl Allylation of Aldehydes with Allylic Alcohols under the Sequential Catalysis of Palladium Complex and Chiral Phosphoric Acid [J]. Acta Chim. Sinica, 2017, 75(12): 1196-1201. |
| [8] | Luo Feihua, Long Yang, Li Zhengkai, Zhou Xiangge. Palladium Catalyzed Arylation of C(sp3)-H Bonds of Carbonyl β-position in Water [J]. Acta Chim. Sinica, 2016, 74(10): 805-810. |
| [9] | Zhou Rong, Xiao Wei, Yin Xiang, Zhan Gu, Chen Yingchun. Diastereo- and Enantioselective [4+2] Cycloadditions of Cyclic Enones with Cyclic 1-Azadienes [J]. Acta Chimica Sinica, 2014, 72(7): 862-866. |
| [10] | Cai Haiting, Li Dandan, Liu Zi, Wang Guanwu. Palladium-Catalyzed Decarboxylative ortho-Acylation of O-Methyl Ketoximes via Direct sp2 C—H Bond Activation [J]. Acta Chimica Sinica, 2013, 71(05): 717-721. |
| [11] | Zhang Binbin, Zhan Dan, Zhang Xiaoping, Xiang Qinjie, Zeng Qingle. Ligand-free Pd-Catalyzed C—N Coupling of Diphenylamine and Aryl Halides under Air [J]. Acta Chimica Sinica, 2012, 70(15): 1655-1659. |
| [12] | NA Lu-Xin, ZHAO Dong-Mei, SONG Shuai, SUN Liang, CHENG Mao-Sheng. Stereochemistry of Narcotine and Phthalideisoquinoline Synthesized via Bischler-Napieralski Reaction [J]. Acta Chimica Sinica, 2011, 69(03): 356-361. |
| [13] | LI Bo-Nan; LIANG Yong; JIAO Lei; HU Li-Bo; DU Da-Ming; XU Jia-Xi*. Synthesis and Stereochemistry of 3-(Thiophen-2-yl)-β-lactams [J]. Acta Chimica Sinica, 2007, 65(16): 1643-1648. |
| [14] | Gao Xiang;Zhang Xiaoyue;Zhang Danwei;Liu Ying;Wu Shihui. A Thermoinduced [3+2] Cycloaddition of Allylic Amines with C_(60) [J]. Acta Chimica Sinica, 2003, 61(10): 1686-1691. |
| [15] | ZHANG JIANJUN;KONG FANZUO. A facile large scale synthesis of the core mannose pentasaccharide of N-linked glycoprotein and its isomer [J]. Acta Chimica Sinica, 2002, 60(1): 150-156. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||