Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (7): 843-856.DOI: 10.6023/A23020040 Previous Articles Next Articles
Review
投稿日期:2023-02-19
发布日期:2023-06-07
作者简介: |
张娜娜, 大连理工大学生物工程专业在读博士生, 研究方向为O-GlcNAc糖基化修饰与肿瘤生物学行为. |
 |
刘宇博, 大连理工大学生命科学与药学学院副教授, 博士生导师, 主要研究方向为化学糖生物学和肿瘤糖生物学. 以化学生物学和糖生物学交叉融合为手段, 开发高效、低毒糖探针, 发展化学糖组学研究新策略. 2014年大连理工大学生物化工专业博士毕业, 以第一作者或通讯作者在Nature Communications, Journal of Biological Chemistry, Cell death & Disease等期刊发表研究论文30余篇, 主持国家自然科学基金3项, 辽宁省自然科学基金3项. |
基金资助:
Nana Zhang, Kairan Yu, Jiting Li, Jianing Zhang, Yubo Liu( )
)
Received:2023-02-19
Published:2023-06-07
Contact:
*E-mail: Supported by:Share
Nana Zhang, Kairan Yu, Jiting Li, Jianing Zhang, Yubo Liu. Application of Chemical Biology to Reveal the Function of O-GlcNAcylation in Diseases: Research Tools and Tactics[J]. Acta Chimica Sinica, 2023, 81(7): 843-856.
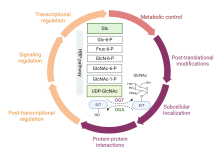

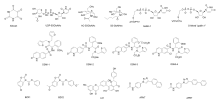

| OGT抑制剂 | 抑制性参数 | 优点 | 缺点 |
|---|---|---|---|
| Alloxan | IC50=100 μmol/L | 细胞渗透性好 | 选择性差 |
| Ac-5SGlcNAc | EC50=5 μmol/L | 选择性好 | 脱靶, 水溶性差 |
| 5SGlcNAc | 与Ac-5SGlcNAc近似 | 选择性好, 细胞渗透性好 | 未知 |
| 5S-GlcNHex | IC50=11 μmol/L | 水溶性、细胞渗透力强 | 同时抑制其它糖基转移酶 |
| Goblin | IC50=18 μmol/L | — | 细胞渗透性差, 无法体内实验 |
| OSMI-1 | IC50=2.7 μmol/L | 细胞渗透性、选择性好 | 降低细胞活力, 脱靶效应 |
| OSMI-2, OSMI-3, OSMI-4 | OSMI-2 IC50= 140 nmol/L OSMI-3 IC50=5 nmol/L OSMI-4 IC50=8 nmol/L | 抑制效果好 | 未知 |
| L01 | IC50=21.8 μmol/L | 细胞可渗透, 天然产物, 低毒性 | 脱靶效应 |
| APNT, APBT | APNT: IC50=66.7 μmol/L APBT IC50=139 μmol/L | 细胞可渗透, 非竞争抑制, 低毒性 | 水中溶解性差, 效力低 |
| LQMed 330 | IC50=11.7 μmol/L | 未知 | 选择性差, 溶解性和渗透性未知 |
| OGT抑制剂 | 抑制性参数 | 优点 | 缺点 |
|---|---|---|---|
| Alloxan | IC50=100 μmol/L | 细胞渗透性好 | 选择性差 |
| Ac-5SGlcNAc | EC50=5 μmol/L | 选择性好 | 脱靶, 水溶性差 |
| 5SGlcNAc | 与Ac-5SGlcNAc近似 | 选择性好, 细胞渗透性好 | 未知 |
| 5S-GlcNHex | IC50=11 μmol/L | 水溶性、细胞渗透力强 | 同时抑制其它糖基转移酶 |
| Goblin | IC50=18 μmol/L | — | 细胞渗透性差, 无法体内实验 |
| OSMI-1 | IC50=2.7 μmol/L | 细胞渗透性、选择性好 | 降低细胞活力, 脱靶效应 |
| OSMI-2, OSMI-3, OSMI-4 | OSMI-2 IC50= 140 nmol/L OSMI-3 IC50=5 nmol/L OSMI-4 IC50=8 nmol/L | 抑制效果好 | 未知 |
| L01 | IC50=21.8 μmol/L | 细胞可渗透, 天然产物, 低毒性 | 脱靶效应 |
| APNT, APBT | APNT: IC50=66.7 μmol/L APBT IC50=139 μmol/L | 细胞可渗透, 非竞争抑制, 低毒性 | 水中溶解性差, 效力低 |
| LQMed 330 | IC50=11.7 μmol/L | 未知 | 选择性差, 溶解性和渗透性未知 |
| O-GlcNAc抗体 | 抗原 | 抗体 | 应用范围 |
|---|---|---|---|
| RL2 | CTSPPDS (O-GlcNAc) SVIVTLLD | IgG | 免疫印迹、免疫荧光、酶联免疫 |
| CTD110.6 | VYKSPVVS (O-GlcNAc) GDTSPRH | IgM | 免疫印迹、免疫组化 |
| HGAC85 | KKFELLPT (O-GlcNAc) PPLSPSRR | IgG | 免疫印迹、免疫组化、免疫沉淀、酶联免疫、免疫细胞化学、染色质免疫沉淀 |
| EPR19847 | CDTSPAAPVS (O-GlcNAc) YADMRTGI | IgG | 免疫印记、免疫沉淀、点杂交 |
| O-GlcNAc抗体 | 抗原 | 抗体 | 应用范围 |
|---|---|---|---|
| RL2 | CTSPPDS (O-GlcNAc) SVIVTLLD | IgG | 免疫印迹、免疫荧光、酶联免疫 |
| CTD110.6 | VYKSPVVS (O-GlcNAc) GDTSPRH | IgM | 免疫印迹、免疫组化 |
| HGAC85 | KKFELLPT (O-GlcNAc) PPLSPSRR | IgG | 免疫印迹、免疫组化、免疫沉淀、酶联免疫、免疫细胞化学、染色质免疫沉淀 |
| EPR19847 | CDTSPAAPVS (O-GlcNAc) YADMRTGI | IgG | 免疫印记、免疫沉淀、点杂交 |
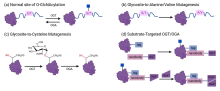

| [1] |
Holt G. D.; Hart G. W. J. Biol. Chem. 1986, 261, 8049.
doi: 10.1016/S0021-9258(19)57510-X |
| [2] |
Shi Q.; Shen Q.; Liu Y. Cancer Cell 2022, 40, 1207.
doi: 10.1016/j.ccell.2022.08.012 |
| [3] |
Hart G. W.; Akimoto Y. The O-GlcNAc Modification, Ed.: Varki, A., New York, 2009, Chapter 18.
|
| [4] |
Peterson S. B.; Hart G. W. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 150.
doi: 10.3109/10409238.2015.1135102 |
| [5] |
Vaidyanathan K.; Wells L. J. Biol. Chem. 2014, 289, 34466.
doi: 10.1074/jbc.R114.591560 |
| [6] |
Akan I.; Olivier-Van Stichelen S.; Bond M. R. J. Neurochem. 2018, 144, 7.
doi: 10.1111/jnc.2018.144.issue-1 |
| [7] |
Banerjee P. S.; Lagerlöf O.; Hart G. W. Mol. Aspects Med. 2016, 51, 1.
doi: 10.1016/j.mam.2016.05.005 |
| [8] |
Nie H.; Yi W. J. Zhejiang Univ. Sci. B 2019, 20, 437.
doi: 10.1631/jzus.B1900150 |
| [9] |
Phueaouan T.; Chaiyawat P.; Netsirisawan P. Oncol. Rep. 2013, 30, 2929.
doi: 10.3892/or.2013.2794 |
| [10] |
Ma Z.; Vocadlo D. J.; Vosseller K. J. Biol. Chem. 2013, 288, 15121.
doi: 10.1074/jbc.M113.470047 |
| [11] |
Jin F. Z.; Yu C.; Zhao D. Z. Exp. Cell Res. 2013, 319, 1482.
doi: 10.1016/j.yexcr.2013.03.013 |
| [12] |
Huang X.; Pan Q.; Sun D. J. Biol. Chem. 2013, 288, 36418.
doi: 10.1074/jbc.M113.495713 |
| [13] |
Shi Y.; Tomic J.; Wen F. Leukemia 2010, 24, 1588.
doi: 10.1038/leu.2010.152 |
| [14] |
Dauphinee S. M.; Ma M.; Too C. K. J. Cell Biochem. 2005, 96, 579.
doi: 10.1002/(ISSN)1097-4644 |
| [15] |
Slawson C.; Hart G. W. Nat. Rev. Cancer 2011, 11, 678.
doi: 10.1038/nrc3114 |
| [16] |
Shafi R.; Iyer S. P.; Ellies L. G. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 5735.
doi: 10.1073/pnas.100471497 |
| [17] |
Yang Y. R.; Song M.; Lee H. Aging Cell 2012, 11, 439.
doi: 10.1111/j.1474-9726.2012.00801.x |
| [18] |
Ferrer C. M.; Lynch T. P.; Sodi V. L. Mol. Cell 2014, 54, 820.
doi: 10.1016/j.molcel.2014.04.026 |
| [19] |
Alteen M. G.; Tan H. Y.; Vocadlo D. J. Curr. Opin. Struct. Biol. 2021, 68, 157.
doi: 10.1016/j.sbi.2020.12.008 |
| [20] |
Wang Y. Acta Chim. Sinica 2013, 71, 1477. (in Chinese)
doi: 10.6023/A13050534 |
|
(王玥, 化学学报, 2013, 71, 1477.)
|
|
| [21] |
Konrad R. J.; Zhang F.; Hale J. E. Biochem. Biophys. Res. Commun. 2002, 293, 207.
doi: 10.1016/S0006-291X(02)00200-0 |
| [22] |
Vibjerg Jensen R.; Johnsen J.; Buus Kristiansen S. Scand Cardiovasc J. 2013, 47, 168.
doi: 10.3109/14017431.2012.756984 |
| [23] |
Borodkin V. S.; Schimpl M.; Gundogdu M. Biochem. J. 2014, 457, 497.
doi: 10.1042/BJ20131272 |
| [24] |
Gloster T. M.; Zandberg W. F.; Heinonen J. E. Nat. Chem. Biol. 2011, 7, 174.
doi: 10.1038/nchembio.520 |
| [25] |
Sodi V. L.; Bacigalupa Z. A.; Ferrer C. M. Oncogene 2018, 37, 924.
doi: 10.1038/onc.2017.395 |
| [26] |
Liu T. W.; Zandberg W. F.; Gloster T. M. Angew. Chem. Int. Ed. 2018, 57, 7644.
doi: 10.1002/anie.v57.26 |
| [27] |
Rafie K.; Gorelik A.; Trapannone R. Bioconjug. Chem. 2018, 29, 1834.
doi: 10.1021/acs.bioconjchem.8b00194 |
| [28] |
Gross B. J.; Kraybill B. C.; Walker S. J. Am. Chem. Soc. 2005, 127, 14588.
doi: 10.1021/ja0555217 |
| [29] |
Jiang J.; Lazarus M. B.; Pasquina L. Nat. Chem. Biol. 2011, 8, 72.
doi: 10.1038/nchembio.711 |
| [30] |
Ortiz-Meoz R. F.; Jiang J.; Lazarus M. B. ACS Chem. Biol. 2015, 10, 1392.
doi: 10.1021/acschembio.5b00004 |
| [31] |
Rahman M. A.; Cho Y.; Hwang H. Brain Sci. 2020, 10, 958.
doi: 10.3390/brainsci10120958 |
| [32] |
Liu Y.; Cao Y.; Pan X. Cell Death Dis. 2018, 9, 485.
doi: 10.1038/s41419-018-0522-0 |
| [33] |
Lee S. J.; Lee D. E.; Choi S. Y. Int. J. Mol. Sci. 2021, 22, 11073.
doi: 10.3390/ijms222011073 |
| [34] |
Lee S. J.; Kwon O. S. Cancers (Basel) 2020, 12, 3154.
doi: 10.3390/cancers12113154 |
| [35] |
Luanpitpong S.; Kang X.; Janan M. Stem Cell Res. Ther. 2022, 13, 274.
doi: 10.1186/s13287-022-02954-5 |
| [36] |
Martin S. E. S.; Tan Z.-W.; Itkonen H. M. J. Am. Chem. Soc. 2018, 140, 13542.
doi: 10.1021/jacs.8b07328 |
| [37] |
Liu X.; Song S.; Chen Z. Acta Biomater. 2022, 151, 148.
doi: 10.1016/j.actbio.2022.08.031 |
| [38] |
Wang Y.; Zhu J.; Zhang L. J. Med. Chem. 2017, 60, 263.
doi: 10.1021/acs.jmedchem.6b01237 |
| [39] |
Liu Y.; Ren Y.; Cao Y. Sci. Rep. 2017, 7, 12334.
doi: 10.1038/s41598-017-12522-0 |
| [40] |
(a) Liu Y. B.; Zhang N. N.; Chen J. J. Chem. J. Chinese Univ. 2018, 39, 1185. (in Chinese)
|
|
(刘宇博, 张娜娜, 陈锦娇, 高等学校化学学报, 2018, 39, 1185.)
|
|
|
(b) Liu X.; Zhang N.; Cao Y. Chin. Pharmacol. Bull. 2020, 36, 1574. (in Chinese)
|
|
|
(刘欣, 张娜娜, 曹禺, 中国药理学通报, 2020, 36, 1574.)
|
|
| [41] |
Zhang N.; Zhu T.; Yu K. Cell Death Dis. 2019, 10, 343.
doi: 10.1038/s41419-019-1577-2 |
| [42] |
Huang H.; Wu Q.; Guo X. J. Cell Physiol. 2021, 236, 7491.
doi: 10.1002/jcp.v236.11 |
| [43] |
Albuquerque S. O.; Barros T. G.; Dias L. R. S. Eur. J. Pharm. Sci. 2020, 154, 105510.
doi: 10.1016/j.ejps.2020.105510 |
| [44] |
Horsch M.; Hoesch L.; Vasella A. Eur. J. Biochem. 1991, 197, 815.
doi: 10.1111/ejb.1991.197.issue-3 |
| [45] |
Laczy B.; Marsh S. A.; Brocks C. A. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1715.
|
| [46] |
Macauley M. S.; Whitworth G. E.; Debowski A. W. J. Biol. Chem. 2005, 280, 25313.
doi: 10.1074/jbc.M413819200 |
| [47] |
Macauley M. S.; He Y.; Gloster T. M. Chem. Biol. 2010, 17, 937.
doi: 10.1016/j.chembiol.2010.07.006 |
| [48] |
Dorfmueller H. C.; Borodkin V. S.; Schimpl M. J. Am. Chem. Soc. 2006, 128, 16484.
doi: 10.1021/ja066743n |
| [49] |
Yuzwa S. A.; Macauley M. S.; Heinonen J. E. Nat. Chem. Biol. 2008, 4, 483.
doi: 10.1038/nchembio.96 |
| [50] |
Hilgers R. H.; Xing D.; Gong K. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H513.
|
| [51] |
Zhu Q.; Zhou H.; Wu L. Nat. Chem. Biol. 2022, 18, 1087.
doi: 10.1038/s41589-022-01085-5 |
| [52] |
Selnick H. G.; Hess J. F.; Tang C. J. Med. Chem. 2019, 62, 10062.
doi: 10.1021/acs.jmedchem.9b01090 |
| [53] |
Yang Y.; Li X.; Luan H. H. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 16616.
doi: 10.1073/pnas.1916121117 |
| [54] |
Martínez-Viturro C. M.; Trabanco A. A.; Royes J. J. Med. Chem. 2020, 63, 14017.
doi: 10.1021/acs.jmedchem.0c01479 |
| [55] |
González-Cuesta M.; Sidhu P.; Ashmus R. A. J. Am. Chem. Soc. 2022, 144, 832.
doi: 10.1021/jacs.1c10504 |
| [56] |
Klein A. L.; Berkaw M. N.; Buse M. G. Mol. Cell. Proteomics 2009, 8, 2733.
doi: 10.1074/mcp.M900207-MCP200 |
| [57] |
Snow C. M.; Senior A.; Gerace L. J. Cell. Biol. 1987, 104, 1143.
doi: 10.1083/jcb.104.5.1143 |
| [58] |
Comer F. I.; Vosseller K.; Wells L. Anal. Biochem. 2001, 293, 169.
doi: 10.1006/abio.2001.5132 |
| [59] |
Turner J. R.; Tartakoff A. M.; Greenspan N. S. Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 5608.
doi: 10.1073/pnas.87.15.5608 |
| [60] |
Yoshida N.; Mortara R. A.; Araguth M. F. Infect Immun, 1989, 57, 1663.
doi: 10.1128/iai.57.6.1663-1667.1989 |
| [61] |
Teo C. F.; Ingale S.; Wolfert M. A. Nat. Chem. Biol. 2010, 6, 338.
doi: 10.1038/nchembio.338 |
| [62] |
Kamemura K.; Hayes B. K.; Comer F. I. J. Biol. Chem. 2002, 277, 19229.
doi: 10.1074/jbc.M201729200 |
| [63] |
Yuzwa S. A.; Yadav A. K.; Skorobogatko Y. Amino Acids 2011, 40, 857.
doi: 10.1007/s00726-010-0705-1 |
| [64] |
Hirosawa M.; Hayakawa K.; Yoneda C. Sci. Rep. 2016, 6, 31785.
doi: 10.1038/srep31785 |
| [65] |
Pathak S.; Borodkin V. S.; Albarbarawi O. Embo J. 2012, 31, 1394.
doi: 10.1038/emboj.2012.8 |
| [66] |
Fujioka K.; Kubota Y.; Takekawa M. Bio-protocol 2018, 8, e3098.
|
| [67] |
Soesanto Y. A.; Luo B.; Jones D. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E974.
|
| [68] |
Diwu Y.; Tian J.; Shi J. J. Tradit. Chin. Med. 2013, 33, 367.
doi: 10.1016/S0254-6272(13)60180-6 |
| [69] |
Ma Z. Y.; Skorobogatko Y.; Vosseller K. Methods Mol. Biol. 2013, 951, 21.
|
| [70] |
Vosseller K.; Trinidad J. C.; Chalkley R. J. Mol. Cell. Proteomics 2006, 5, 923.
doi: 10.1074/mcp.T500040-MCP200 |
| [71] |
Liu W.; Han G.; Yin Y. Glycobiology 2018, 28, 363.
doi: 10.1093/glycob/cwy029 |
| [72] |
Su Y.; Ye X.; Xu B. Glycobiology 2020, 30, 159.
|
| [73] |
Schimpl M.; Borodkin V. S.; Gray L. J. Chem. Biol. 2012, 19, 173.
doi: 10.1016/j.chembiol.2012.01.011 |
| [74] |
Mariappa D.; Selvan N.; Borodkin V. Biochem. J. 2015, 470, 255.
doi: 10.1042/BJ20150610 |
| [75] |
Selvan N.; Williamson R.; Mariappa D. Nat. Chem. Biol. 2017, 13, 882.
doi: 10.1038/nchembio.2404 |
| [76] |
Song J.; Liu C.; Wang X. ACS Chem. Biol. 2021, 16, 1040.
doi: 10.1021/acschembio.1c00185 |
| [77] |
Isono T. PLOS ONE 2011, 6, e18959.
|
| [78] |
Gilormini P. A.; Batt A. R.; Pratt M. R. Chem. Sci. 2018, 9, 7585.
doi: 10.1039/C8SC02241K |
| [79] |
Cheng B.; Tang Q.; Zhang C. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2021, 14, 363.
doi: 10.1146/anchem.2021.14.issue-1 |
| [80] |
Vocadlo D. J.; Hang H. C.; Kim E. J. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 9116.
doi: 10.1073/pnas.1632821100 |
| [81] |
Yu S. H.; Boyce M.; Wands A. M. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 4834.
doi: 10.1073/pnas.1114356109 |
| [82] |
Zhu Y.; Wu J.; Chen X. Angew. Chem. Int. Ed. 2016, 55, 9301.
doi: 10.1002/anie.v55.32 |
| [83] |
Hang H. C.; Yu C.; Kato D. L. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 14846.
doi: 10.1073/pnas.2335201100 |
| [84] |
Boyce M.; Carrico I. S.; Ganguli A. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 3141.
doi: 10.1073/pnas.1010045108 |
| [85] |
Xu S.; Zheng J.; Xiao H. Anal. Chem. 2022, 94, 3343.
doi: 10.1021/acs.analchem.1c05438 |
| [86] |
Zhu Y.; Willems L. I.; Salas D. J. Am. Chem. Soc. 2020, 142, 15729.
doi: 10.1021/jacs.0c04121 |
| [87] |
Lin W.; Gao L.; Chen X. ChemBioChem 2015, 16, 2571.
doi: 10.1002/cbic.201500544 |
| [88] |
Qin W.; Qin K.; Fan X. Angew. Chem. Int. Ed. 2018, 57, 1817.
doi: 10.1002/anie.201711710 |
| [89] |
Hao Y.; Fan X.; Shi Y. Nat. Commun. 2019, 10, 4065.
doi: 10.1038/s41467-019-11942-y |
| [90] |
Qin K.; Zhang H.; Zhao Z. J. Am. Chem. Soc. 2020, 142, 9382.
doi: 10.1021/jacs.0c02110 |
| [91] |
Zaro B. W.; Yang Y. Y.; Hang H. C. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 8146.
doi: 10.1073/pnas.1102458108 |
| [92] |
Chuh K. N.; Zaro B. W.; Piller F. J. Am. Chem. Soc. 2014, 136, 12283.
doi: 10.1021/ja504063c |
| [93] |
Chuh K. N.; Batt A. R.; Zaro B. W. J. Am. Chem. Soc. 2017, 139, 7872.
doi: 10.1021/jacs.7b02213 |
| [94] |
Darabedian N.; Gao J.; Chuh K. N. J. Am. Chem. Soc. 2018, 140, 7092.
doi: 10.1021/jacs.7b13488 |
| [95] |
Pedowitz N. J.; Jackson E. G.; Overhulse J. M. ACS Chem. Biol. 2021, 16, 1924.
doi: 10.1021/acschembio.1c00470 |
| [96] |
Lin W.; Gao L.; Chen X. Chembiochem 2015, 16, 2571.
doi: 10.1002/cbic.201500544 |
| [97] |
Torres C. R.; Hart G. W. J. Biol. Chem. 1984, 259, 3308.
doi: 10.1016/S0021-9258(17)43295-9 |
| [98] |
Khidekel N.; Arndt S.; Lamarre-Vincent N. J. Am. Chem. Soc. 2003, 125, 16162.
doi: 10.1021/ja038545r |
| [99] |
Clark P. M.; Dweck J. F.; Mason D. E. J. Am. Chem. Soc. 2008, 130, 11576.
doi: 10.1021/ja8030467 |
| [100] |
Balana A. T.; Mukherjee A.; Nagpal H. J. Am. Chem. Soc. 2021, 143, 16030.
doi: 10.1021/jacs.1c06192 |
| [101] |
Khidekel N.; Ficarro S. B.; Peters E. C. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 13132.
doi: 10.1073/pnas.0403471101 |
| [102] |
Aguilar A. L.; Hou X.; Wen L. Chembiochem 2017, 18, 2416.
doi: 10.1002/cbic.v18.24 |
| [103] |
Tian Y.; Zhu Q.; Sun Z. Angew. Chem. Int. Ed. 2021, 60, 26128.
doi: 10.1002/anie.v60.50 |
| [104] |
Chen Y.; Tang F.; Qin H. Angew. Chem. Int. Ed. 2022, 61, e202117849.
|
| [105] |
Rexach J. E.; Rogers C. J.; Yu S. H. Nat. Chem. Biol. 2010, 6, 645.
doi: 10.1038/nchembio.412 |
| [106] |
Darabedian N.; Thompson J. W.; Chuh K. N. Biochemistry 2018, 57, 5769.
doi: 10.1021/acs.biochem.8b00648 |
| [107] |
Qin W.; Lv P.; Fan X. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E6749.
|
| [108] |
Ma J.; Hart G. W. Clin. Proteomics 2014, 11, 8.
doi: 10.1186/1559-0275-11-8 |
| [109] |
Thompson J. W.; Sorum A. W.; Hsieh-Wilson L. C. Biochemistry 2018, 57, 4010.
doi: 10.1021/acs.biochem.8b00516 |
| [110] |
Maynard J. C.; Chalkley R. J. Mol. Cell. Proteomics 2021, 20, 100031.
doi: 10.1074/mcp.R120.002206 |
| [111] |
Ma J.; Wu C.; Hart G. W. Chem. Rev. 2021, 121, 1513.
doi: 10.1021/acs.chemrev.0c00884 |
| [112] |
Li Y. Y.; Peng Y.; Lu H. J. Acta Chim. Sinica 2021, 79, 705. (in Chinese)
doi: 10.6023/A21020048 |
|
(李月悦, 彭叶, 陆豪杰, 化学学报, 2021, 79, 705.)
|
|
| [113] |
Xu S.; Tong M.; Suttapitugsakul S. Cell Rep. 2022, 39, 110946.
doi: 10.1016/j.celrep.2022.110946 |
| [114] |
Hahne H.; Sobotzki N.; Nyberg T. J. Proteome Res. 2013, 12, 927.
doi: 10.1021/pr300967y |
| [115] |
Liu J.; Shao X.; Qin W. Cell Chem. Biol. 2021, 28, 788.
doi: 10.1016/j.chembiol.2021.01.024 |
| [116] |
Liu Y.; Chen Q.; Zhang N. Nat. Commun. 2020, 11, 5898.
doi: 10.1038/s41467-020-19579-y |
| [117] |
He J.; Fan Z.; Tian Y. J. Am. Chem. Soc. 2022, 144, 4289.
doi: 10.1021/jacs.1c11041 |
| [118] |
Liu Y.; Nelson Z. M.; Reda A. ACS Chem. Biol. 2022, 17, 2153.
doi: 10.1021/acschembio.2c00282 |
| [119] |
Banerjee P. S.; Hart G. W.; Cho J. W. Chem. Soc. Rev. 2013, 42, 4345.
doi: 10.1039/C2CS35412H |
| [120] |
Wulff-Fuentes E.; Berendt R. R.; Massman L. Sci. Data 2021, 8, 25.
doi: 10.1038/s41597-021-00810-4 |
| [121] |
Ma J.; Li Y.; Hou C. Glycobiology 2021, 31, 719.
doi: 10.1093/glycob/cwab003 |
| [122] |
Woo C. M.; Lund P. J.; Huang A. C. Mol. Cell. Proteomics 2018, 17, 764.
doi: 10.1074/mcp.RA117.000261 |
| [123] |
Zhao P.; Viner R.; Teo C. F. J. Proteome Res. 2011, 10, 4088.
doi: 10.1021/pr2002726 |
| [124] |
Zhang Y.; Xie X.; Zhao X. J. Proteomics 2018, 170, 14.
doi: 10.1016/j.jprot.2017.09.014 |
| [125] |
Marino F.; Bern M.; Mommen G. P. M. J. Am. Chem. Soc. 2015, 137, 10922.
doi: 10.1021/jacs.5b06586 |
| [126] |
Liu J.; Hao Y.; He Y. ACS Chem. Biol. 2021, 16, 1917.
doi: 10.1021/acschembio.1c00301 |
| [127] |
Santala V.; Saviranta P. J. Immunol. Methods 2004, 284, 159.
doi: 10.1016/j.jim.2003.10.013 |
| [128] |
Wang Z.; Udeshi N. D.; O'Malley M. Mol. Cell. Proteomics 2010, 9, 153.
doi: 10.1074/mcp.M900268-MCP200 |
| [129] |
Li J.; Li Z.; Duan X. ACS Chem. Biol. 2019, 14, 4.
doi: 10.1021/acschembio.8b01052 |
| [130] |
Khidekel N.; Ficarro S. B.; Clark P. M. Nat. Chem. Biol. 2007, 3, 339.
doi: 10.1038/nchembio881 |
| [131] |
Woo C. M.; Iavarone A. T.; Spiciarich D. R. Nat. Methods 2015, 12, 561.
doi: 10.1038/nmeth.3366 |
| [132] |
Wang S.; Yang F.; Petyuk V. A. J. Pathol. 2017, 243, 78.
doi: 10.1002/path.2017.243.issue-1 |
| [133] |
Liu J.; Hao Y.; Wang C. ACS Chem. Biol. 2022, 17, 513.
doi: 10.1021/acschembio.1c00981 |
| [134] |
Frenkel-Pinter M.; Richman M.; Belostozky A. Chemistry 2016, 22, 5945.
|
| [135] |
Levine P. M.; Balana A. T.; Sturchler E. J. Am. Chem. Soc. 2019, 141, 14210.
doi: 10.1021/jacs.9b05365 |
| [136] |
Lv P.; Du Y.; He C. Nat. Chem. 2022, 14, 831.
doi: 10.1038/s41557-022-00946-9 |
| [137] |
Li J.; Li Z.; Duan X. ACS Chem. Biol. 2019, 14, 4.
doi: 10.1021/acschembio.8b01052 |
| [138] |
Yi W.; Clark P. M.; Mason D. E. Science 2012, 337, 975.
doi: 10.1126/science.1222278 |
| [139] |
Yang W. H.; Kim J. E.; Nam H. W. Nat. Cell Biol. 2006, 8, 1074.
doi: 10.1038/ncb1470 |
| [140] |
Gorelik A.; Bartual S. G.; Borodkin V. S. Nat. Struct. Mol. Biol. 2019, 26, 1071.
doi: 10.1038/s41594-019-0325-8 |
| [141] |
Maynard J. C.; Burlingame A. L.; Medzihradszky K. F. Mol. Cell. Proteomics 2016, 15, 3405.
doi: 10.1074/mcp.M116.061549 |
| [142] |
Macauley M. S.; Stubbs K. A.; Vocadlo D. J. J. Am. Chem. Soc. 2005, 127, 17202.
doi: 10.1021/ja0567687 |
| [143] |
Tegl G.; Hanson J.; Chen H. M. Angew. Chem. Int. Ed. 2019, 58, 1632.
doi: 10.1002/anie.v58.6 |
| [144] |
Ramirez D. H.; Aonbangkhen C.; Wu H. Y. ACS Chem. Biol. 2020, 15, 1059.
doi: 10.1021/acschembio.0c00074 |
| [145] |
Ge Y.; Ramirez D. H.; Yang B. Nat. Chem. Biol. 2021, 17, 593.
doi: 10.1038/s41589-021-00757-y |
| [146] |
Gupta R.; Brunak S. Pac Symp Biocomput 2002, 310.
|
| [147] |
Hamby S. E.; Hirst J. D. BMC Bioinformatics 2008, 9, 500.
doi: 10.1186/1471-2105-9-500 |
| [148] |
Hornbeck P. V.; Kornhauser J. M.; Latham V. Nucleic Acids Res. 2019, 47, D433.
|
| [149] |
York W. S.; Mazumder R.; Ranzinger R. Glycobiology 2020, 30, 72.
doi: 10.1093/glycob/cwz080 |
| [150] |
Huang K. Y.; Lee T. Y.; Kao H. J. Nucleic Acids Res. 2019, 47, D298.
|
| [151] |
Cekic N.; Heinonen J. E.; Stubbs K. A. Chem. Sci. 2016, 7, 3742.
doi: 10.1039/C6SC00370B |
| [1] | Zhang Lixia, Du Xiufang, Zeng Ying. Chemistry in Separation and Enrichment of Glycoproteins/Glycopeptides [J]. Acta Chim. Sinica, 2016, 74(2): 149-154. |
| [2] | Hu Zhengyan, Sun Zhen, Zhang Yi, Wu Ren’an, Zou Hanfa. Glycoproteome Quantification of Human Lung Cancer Cells Exposed to Amorphous Silica Nanoparticles [J]. Acta Chimica Sinica, 2012, 70(19): 2059-2065. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||