Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (9): 1135-1141.DOI: 10.6023/A23040184 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Article
刘士琨a, 邓程维b, 姬峰b, 闵宇霖a,*( ), 李和兴a,*(
), 李和兴a,*( )
)
投稿日期:2023-04-29
发布日期:2023-07-13
作者简介:基金资助:
Shikun Liua, Chengwei Dengb, Feng Jib, Yulin Mina( ), Hexing Lia(
), Hexing Lia( )
)
Received:2023-04-29
Published:2023-07-13
Contact:
*E-mail: About author:Supported by:Share
Shikun Liu, Chengwei Deng, Feng Ji, Yulin Min, Hexing Li. Design and Study on Pore Structure of Cathode Double Catalytic Layer in High-temperature Proton Exchange Membrane Fuel Cell★[J]. Acta Chimica Sinica, 2023, 81(9): 1135-1141.
| 内CL | 外CL | ||||
|---|---|---|---|---|---|
| Pt载量/ (mg•cm-2) | 造孔剂质量∶总固体质量/% | Pt载量/ (mg•cm-2) | Pt载量/ (mg•cm-2) | ||
| MEA 0 | 0.250 | 0 | 0.253 | 0.250 | |
| MEA 1 | 0.252 | 0 | 0.248 | 0.252 | |
| MEA 2 | 0.251 | 0 | 0.248 | 0.251 | |
| MEA 3 | 0.250 | 0 | 0.251 | 0.250 | |
| MEA 4 | 0.249 | 10 | 0.253 | 0.249 | |
| MEA 5 | 0.252 | 10 | 0.251 | 0.252 | |
| MEA 6 | 0.250 | 10 | 0.252 | 0.250 | |
| 内CL | 外CL | ||||
|---|---|---|---|---|---|
| Pt载量/ (mg•cm-2) | 造孔剂质量∶总固体质量/% | Pt载量/ (mg•cm-2) | Pt载量/ (mg•cm-2) | ||
| MEA 0 | 0.250 | 0 | 0.253 | 0.250 | |
| MEA 1 | 0.252 | 0 | 0.248 | 0.252 | |
| MEA 2 | 0.251 | 0 | 0.248 | 0.251 | |
| MEA 3 | 0.250 | 0 | 0.251 | 0.250 | |
| MEA 4 | 0.249 | 10 | 0.253 | 0.249 | |
| MEA 5 | 0.252 | 10 | 0.251 | 0.252 | |
| MEA 6 | 0.250 | 10 | 0.252 | 0.250 | |






| 电压/V | 最大功率密度/ (mW•cm-2) | |||||
|---|---|---|---|---|---|---|
| 0.2 A•cm-2 | 0.5 A•cm-2 | 1.2 A•cm-2 | ||||
| MEA 0 | 0.618 | 0.507 | 0.306 | 370 | ||
| MEA 1 | 0.615 | 0.518 | 0.352 | 425 | ||
| MEA 2 | 0.632 | 0.531 | 0.366 | 439 | ||
| MEA 3 | 0.605 | 0.500 | 0.301 | 363 | ||
| MEA 4 | 0.619 | 0.514 | 0.316 | 379 | ||
| MEA 5 | 0.606 | 0.523 | 0.359 | 432 | ||
| MEA 6 | 0.620 | 0.521 | 0.342 | 410 | ||
| 电压/V | 最大功率密度/ (mW•cm-2) | |||||
|---|---|---|---|---|---|---|
| 0.2 A•cm-2 | 0.5 A•cm-2 | 1.2 A•cm-2 | ||||
| MEA 0 | 0.618 | 0.507 | 0.306 | 370 | ||
| MEA 1 | 0.615 | 0.518 | 0.352 | 425 | ||
| MEA 2 | 0.632 | 0.531 | 0.366 | 439 | ||
| MEA 3 | 0.605 | 0.500 | 0.301 | 363 | ||
| MEA 4 | 0.619 | 0.514 | 0.316 | 379 | ||
| MEA 5 | 0.606 | 0.523 | 0.359 | 432 | ||
| MEA 6 | 0.620 | 0.521 | 0.342 | 410 | ||

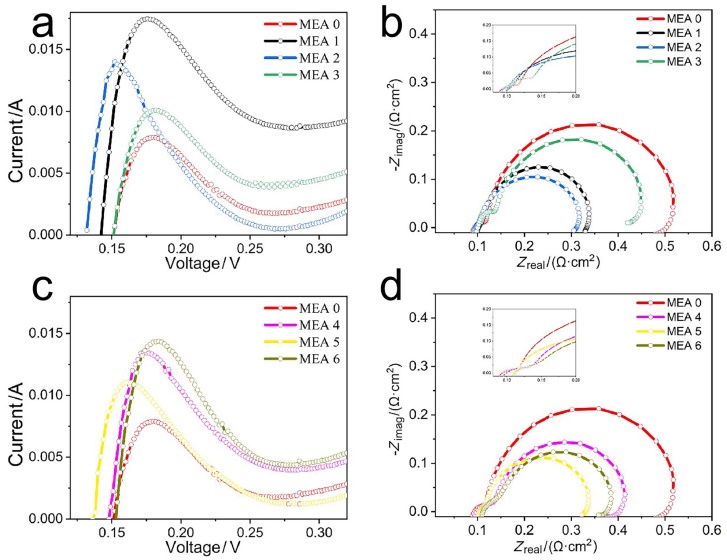
| RΩ/(mΩ•cm2) | Rp/(mΩ•cm2) | (Rct+Rmt)/(mΩ•cm2) | |
|---|---|---|---|
| MEA 0 | 0.091 | 0.038 | 0.388 |
| MEA 1 | 0.094 | 0.018 | 0.24 |
| MEA 2 | 0.099 | 0.021 | 0.204 |
| MEA 3 | 0.112 | 0.043 | 0.303 |
| MEA 4 | 0.108 | 0.049 | 0.264 |
| MEA 5 | 0.112 | 0.025 | 0.214 |
| MEA 6 | 0.118 | 0.046 | 0.221 |
| RΩ/(mΩ•cm2) | Rp/(mΩ•cm2) | (Rct+Rmt)/(mΩ•cm2) | |
|---|---|---|---|
| MEA 0 | 0.091 | 0.038 | 0.388 |
| MEA 1 | 0.094 | 0.018 | 0.24 |
| MEA 2 | 0.099 | 0.021 | 0.204 |
| MEA 3 | 0.112 | 0.043 | 0.303 |
| MEA 4 | 0.108 | 0.049 | 0.264 |
| MEA 5 | 0.112 | 0.025 | 0.214 |
| MEA 6 | 0.118 | 0.046 | 0.221 |
| [1] |
Xiao, F.; Wang, Y.; Wu, Z.; Chen, G.; Yang, F.; Zhu, S.; Shao, M. Adv. Mater. 2021, 33, 2006292.
doi: 10.1002/adma.v33.50 |
| [2] |
Tang, M. H.; Zhang, S. M.; Chen, S. L. Chem. Soc. Rev. 2022, 51, 1529.
doi: 10.1039/D1CS00981H |
| [3] |
Chen, J. N.; Bailey, J. J.; Britnell, L.; Maria, P. P.; Zhang, Z.; Strudwick, A.; Hack, J.; Guo, Z. M.; Martin, P.; Dan, J. L.; Stuart, M. H. Nano Energy 2022, 93, 106829.
doi: 10.1016/j.nanoen.2021.106829 |
| [4] |
Hong, L. H.; Wang, B. L.; Zhao, C. G. Appl. Surf. Sci. 2019, 31, 785.
|
| [5] |
Mazúr, P.; Soukup, J.; Paidar, M.; Bouzek, K. J. Appl. Electrochem. 2011, 41, 1013.
doi: 10.1007/s10800-011-0325-9 |
| [6] |
Yurko, Y.; Elbaz, L. Electrochim. Acta 2021, 389, 138676.
doi: 10.1016/j.electacta.2021.138676 |
| [7] |
Tian, X. L.; Xu, Y. Y.; Zhang, W. Y.; Wu, T.; Xia, B. Y.; Wang, X. ACS Energy Lett. 2017, 2, 2035.
doi: 10.1021/acsenergylett.7b00593 |
| [8] |
Wannek, C.; Lehnert, W.; Mergel, J. J. Power Sources 2009, 192, 258.
doi: 10.1016/j.jpowsour.2009.03.051 |
| [9] |
Yin, Y.; Liu, J.; Chang, Y. F.; Zhu, Y. Z.; Xie, X.; Qin, Y. Z.; Zhang, J. F.; Jiao, K.; Guiver, M. D. Electrochim. Acta 2018, 296, 450.
doi: 10.1016/j.electacta.2018.11.048 |
| [10] |
Ji, Z. Q.; Chen, J. N.; Guo, Z. M.; Zhao, Z. Y.; Cai, R. S.; Rigby, T. P.; Sarah, J.; Shen, Y. T.; Holmes, S. M. J. Energy Chem. 2022, 75, 399.
doi: 10.1016/j.jechem.2022.08.004 |
| [11] |
Li, W. W.; Shang, Y. M.; Wang, S. B.; Xie, X. F.; Lu, Y. F. Chem. Eng. J. 2011, 62, 131. (in Chinese)
|
|
(李微微, 尚玉明, 王树博, 谢晓峰, 吕亚非, 化工学报, 2011, 62, 131.)
|
|
| [12] |
Lee, E.; Kim, D. H.; Pak, C. H. Appl. Surf. Sci. 2020, 510, 145461.
doi: 10.1016/j.apsusc.2020.145461 |
| [13] |
Kazeminasab, B.; Rowshanzamir, S.; Ghadamian, H. Korean J. Chem. Eng. 2017, 34, 2978.
doi: 10.1007/s11814-017-0202-2 |
| [14] |
Barron, O.; Su, H. N.; Linkov, V.; Pollet, B. G.; Pasupathi, S. K. J. Power Sources 2015, 278, 718.
doi: 10.1016/j.jpowsour.2014.12.139 |
| [15] |
Gao, C. M.; Hu, M. S.; Wang, L.; Wang, L. Polymers 2020, 12, 515.
doi: 10.3390/polym12030515 |
| [16] |
Sun, M.; Huang, J. C.; Xia, Z. G.; Wang, S. L.; Sun, G. G. AIChE J. 2022, 68, 8.
|
| [17] |
Kim, S.; Yuk, S.; Kim, H. G.; Choi, C. Y.; Kim, R. Y.; Lee, J. Y.; Hong, Y. T.; Kim, H. T. J. Mater. 2017, 5, 33.
|
| [18] |
Seland, F.; Berning, T.; Børresen, B.; Tunold, R. J. Power Sources 2006, 160, 1.
doi: 10.1016/j.jpowsour.2005.12.081 |
| [19] |
Kim, G. H.; Eom, S.; Kim, M. J.; Yoo, S. J.; Jang, J. H.; Kim, H.; Cho, E. ACS Appl. Mater. Interfaces 2015, 7, 27581.
doi: 10.1021/acsami.5b07346 |
| [20] |
Su, H. N.; Liang, H. G.; Bladergroen, J.; Linkov, V.; Pollet, B. G.; Pasupathi, S. J. Electrochem. 2014, 161, 4.
|
| [21] |
Liu, S.; Klaus, W.; Werner, L. Int. J. Hydrog. 2021, 46, 27.
|
| [22] |
Zhang, J. J.; Wang, H. N.; Li, W.; Zhang, J.; Lu, D.; Yan, W.; Xiang, Y.; Lu, S. F. J. Power Sources 2021, 505, 230059.
doi: 10.1016/j.jpowsour.2021.230059 |
| [23] |
Bender, G.; Zawodzinski, T.; Saab, A. P. J. Power Sources 2003, 124, 114.
doi: 10.1016/S0378-7753(03)00735-3 |
| [24] |
Chung, Y. H.; Kim, S. J.; Chung, D. Y.; Park, H. Y.; Sung, Y. E.; Yoo, S.; Jang, J. ChemComm. 2015, 51, 2968.
|
| [25] |
Chao, G.; Tang, H. Y.; Ju, Q.; Li, N. W.; Geng, K. J. Power Sources 2023, 556, 232473.
doi: 10.1016/j.jpowsour.2022.232473 |
| [26] |
Yoon, Y. G.; Yang, T. H.; Park, G. G.; Lee, W. Y.; Kim, C. S. J. Power Sources 2003, 118, 189.
doi: 10.1016/S0378-7753(03)00092-2 |
| [27] |
Su, H. N.; Jao, T. C.; Pasupathi, S.; Bladergroen, B. J.; Linkov, V.; Pollet, B. G. J. Power Sources 2014, 246, 63.
doi: 10.1016/j.jpowsour.2013.07.062 |
| [28] |
Garsany, Y.; Atkinson, R. W.; Gould, R. D.; Martin, R.; Dubau, L.; Chatenet, M.; Swider, K. M. J. Power Sources 2021, 514, 230514.
|
| [29] |
Zlotorowicz, A.; Jayasayee, K.; Dahl, P. I.; Thomassen, M. S.; Kjelstrup, S. J. Power Sources 2015, 287, 472.
doi: 10.1016/j.jpowsour.2015.04.079 |
| [30] |
Zhao, J.; He, X.; Wang, L.; Tian, J.; Wan, C.; Jiang, C. Int. J. Hydrog. 2007, 32, 380.
doi: 10.1016/j.ijhydene.2006.06.057 |
| [31] |
Tian, J. H.; Liu, B. W.; Liu, X.; Zhu, K.; Chen, Y. X. PST. 2005, 03, 154. (in Chinese)
|
|
(田建华, 刘邦卫, 刘翔, 朱科, 陈延禧, 化学电源, 2005, 03, 154.)
|
|
| [32] |
Gloaguen, F.; Convert, P.; Gamburzev, S.; Velev, O. A.; Srinivasan, S. Electrochim. Acta 1998, 43, 3767.
doi: 10.1016/S0013-4686(98)00136-4 |
| [33] |
Cui, L. R.; Zhang, J.; Sun, Y. Y.; Lu, S. F.; Xiang, Y. Acta Chim. Sinica 2019, 77, 47. (in Chinese)
doi: 10.6023/A18080344 |
|
(崔丽瑞, 张劲, 孙一焱, 卢善富, 相艳, 化学学报, 2019, 77, 47.)
doi: 10.6023/A18080344 |
|
| [34] |
Wang, M.; Chen, M.; Yang, Z. Y.; Liu, G. C.; Lee, J. K.; Yang, W.; Wang, X. D. Energy Convers. Manag. 2019, 191, 132.
doi: 10.1016/j.enconman.2019.04.014 |
| [35] |
Ji, F.; Zheng, B. W.; Luo, R. Y.; Du, W.; Deng, C. W.; Yang, S.; Liu, Z. Q. Chem. Ind. Eng. Prog. 2022, 41, 5325. (in Chinese)
|
|
(姬峰, 郑博文, 罗若尹, 杜玮, 邓呈维, 杨声, 刘志强, 化工进展, 2022, 41, 5325.)
doi: 10.16085/j.issn.1000-6613.2021-2558 |
|
| [36] |
Bevilacqua, N.; Schmid, M.; Zeis, R. Power Sources. 2020, 471, 228469.
doi: 10.1016/j.jpowsour.2020.228469 |
| [37] |
Zhang, W. Q.; Yao, D. G.; Tian, L. L.; Xie, Z.; Ma, Q.; Xu, Q.; Pasupathi, S.; Xing, L.; Su, H. N. J. Taiwan Inst. Chem. Eng. 2021, 125, 258.
|
| [38] |
Wang, H. S.; Chih, P. H.; Shiuan, W. S.; Tseng, Y. J.; Gang, F. J. Am. Chem. Soc. 2012, 243, 529.
|
| [39] |
Zhang, S. M. Ph.D. Dissertation, Zhejiang University, Hangzhou, 2023. (in Chinese)
|
|
(张硕猛, 博士论文, 浙江大学, 杭州, 2023.)
|
|
| [40] |
Duan, K. J.; Roswitha, Z.; Sui, B. J. J. Chongqing Univ. 2022, 45, 34. (in Chinese)
|
|
(段康俊, Roswitha, Z., 隋邦傑, 重庆大学学报, 2022, 45, 34.)
|
|
| [41] |
Bjorn, W. J.; Douglas, R. M. Energy Environ. Sci. 2011, 4, 2790.
doi: 10.1039/c0ee00652a |
| [42] |
Li, T. Y.; Wang, K. J.; Wang, J. H.; Liu, Y. Q.; Han, Y. F.; Song, J. H.; Hu, H. W.; Lin, G. G.; Liu, Y. Mater. Sci. 2020, 55, 4558.
doi: 10.1007/s10853-019-04323-9 |
| [43] |
Eunae, L.; Do-Hyung, K.; Chanho, P. Appl. Surf. Sci. 2020, 510, 13242.
|
| [1] | Ping Li, Qiyu Yang, Jing Zeng, Ran Zhang, Qiuyan Chen, Fei Yan. Effect of Fluorine Doping on the Performance of Reversible Solid Oxide Cells and Related Kinetic Studies [J]. Acta Chimica Sinica, 2024, 82(1): 36-45. |
| [2] | Yanli Li, Dandan Yu, Sen Lin, Dongfei Sun, Ziqiang Lei. Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries [J]. Acta Chimica Sinica, 2021, 79(2): 200-207. |
| [3] | Qimei Liang, Yujiao Guo, Junming Guo, Mingwu Xiang, Xiaofang Liu, Wei Bai, Ping Ning. Preparation and High Temperature Electrochemical Performance of LiNi0.08Mn1.92O4 Cathode Material of Submicron Truncated Octahedron [J]. Acta Chimica Sinica, 2021, 79(12): 1526-1533. |
| [4] | Song Xuexi, Li Jicheng, Li Zhaohui, Li Xifei, Ding Yanhuai, Xiao Qizhen, Lei Gangtie. Effect of K-Doping on the Sodium-storage Performance of Sodium Vanadate Nanoplates [J]. Acta Chim. Sinica, 2019, 77(7): 625-633. |
| [5] | Chang Shilei, Liang Feng, Yao Yaochun, Ma Wenhui, Yang Bin, Dai Yongnian. Research Progress of Metallic Carbon Dioxide Batteries [J]. Acta Chim. Sinica, 2018, 76(7): 515-525. |
| [6] | Liu Qingchao, Ma Shiyu, Xu Jijing, Li Zhongjun, Zhang Xinbo. Design and Preparation of Advanced Materials for Lithium-Air Batteries [J]. Acta Chimica Sinica, 2017, 75(2): 137-146. |
| [7] | Yang Chun, Gong Zhengliang, Zhao Wengao, Yang Yong. Synthesis and Electrochemical Performance of Lithium Rich Cathode Materials xLi3NbO4·(1-x)LiMO2 (M=Mn, Co; 0 < x < 1) for Li-ion Batteries [J]. Acta Chim. Sinica, 2017, 75(2): 212-217. |
| [8] | Kong Lijuan, Zhou Xiaoyan, Fan Saiying, Li Zaijun, Gu Zhiguo. Study on the Synthesis and Electrochemical Performance of Histidine-Functionalized Graphene Quantum Dots@Silicon Composite Anode Material [J]. Acta Chim. Sinica, 2016, 74(7): 620-628. |
| [9] | Liu Xin, Xie Jingying, Zhao Hailei, Wang Ke, Tang Weiping, Pan Yanlin, Feng Zhenhe, Lv Pengpeng. Synthesis and Properties of Sn30Co30C40 Ternary Alloy Anode Material for Lithium Ion Battery [J]. Acta Chimica Sinica, 2013, 71(07): 1011-1016. |
| [10] | Zuo Xiaoxi, Li Qi, Liu Jiansheng, Xiao Xin, Fan Chengjie, Nan Junmin. Preparation and Performances of Room Molten Salt as Electrolyte in Carbon-carbon Capacitor Based on LiPF6 and Trifluoroacetamide [J]. Acta Chimica Sinica, 2012, 70(04): 367-371. |
| [11] | XIA Xiao-Hong, SHI Lei, HE Yue-De, YANG Li, LIU Hong-Bo. Effect of Carbonization Temperature on Pore Structure and Electrochemical Properties of Tobacco Stem-based Activated Carbon [J]. Acta Chimica Sinica, 2011, 69(21): 2627-2631. |
| [12] | JIN Xiao-Jing, HU Zhong-Ai, XIE Chi-Jing, FU Guo-Rui, ZHANG Zi-Yu, YANG Yu-Yang. Synthesis of Disk-like α-Co(OH)2 and Its Electrochemical Performances [J]. Acta Chimica Sinica, 2010, 68(9): 845-850. |
| [13] | LIANG Ying, LIU Hua-Jun, LU Jun, TIAN Zhi-Gao. Hydrothermal Synthesis and Electrochemical Performance of Bi2O3 Nano-sheets [J]. Acta Chimica Sinica, 2010, 68(19): 1977-1980. |
| [14] | . Electrochemical Performance of New-type Electrode Material Ni(OH)2.05 [J]. Acta Chimica Sinica, 2009, 67(12): 1343-1348. |
| [15] | ZHANG Ying LIU Kai-Yu*,1 ZHANG Wei1,2 WANG Hong-En. Electrochemical Performance of Electrodes in MnO2 Supercapacitor [J]. Acta Chimica Sinica, 2008, 66(8): 909-913. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||