Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (11): 1471-1477.DOI: 10.6023/A23050202 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Article
杨镇鸿, 干晓娟, 王书哲, 段君元, 翟天佑, 刘友文*( )
)
投稿日期:2023-05-05
发布日期:2023-08-14
作者简介:基金资助:
Zhenhong Yang, Xiaojuan Gan, Shuzhe Wang, Junyuan Duan, Tianyou Zhai, Youwen Liu( )
)
Received:2023-05-05
Published:2023-08-14
Contact:
*E-mail: About author:Supported by:Share
Zhenhong Yang, Xiaojuan Gan, Shuzhe Wang, Junyuan Duan, Tianyou Zhai, Youwen Liu. Preparation of Metallic Ni3N Nanoparticles and Its Electrooxidation Performance for Ethylene Glycol★[J]. Acta Chimica Sinica, 2023, 81(11): 1471-1477.


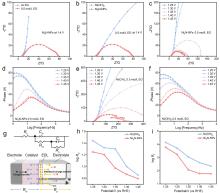

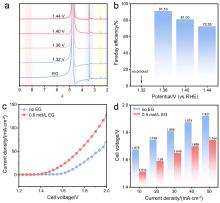

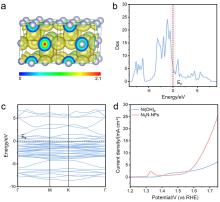

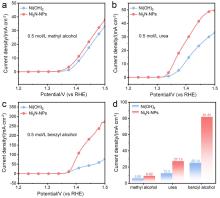

| [1] |
Chatenet M.; Pollet B. G.; Dekel D. R.; Dionigi F.; Deseure J.; Millet P.; Braatz R. D.; Bazant M. Z.; Eikerling M.; Staffell I.; Balcombe P.; Shao-Horn Y.; Schafer H. Chem. Soc. Rev. 2022, 51, 4583.
doi: 10.1039/d0cs01079k pmid: 35575644 |
| [2] |
Zhan F.; Mebrahtu C.; Liao L. F.; Palkovits R.; Beine A. K. J. Energy Chem. 2022, 69, 301.
doi: 10.1016/j.jechem.2022.01.025 |
| [3] |
Jiao Y.; Zheng Y.; Jaroniec M.; Qiao S. Z. Chem. Soc. Rev. 2015, 44, 2060.
doi: 10.1039/c4cs00470a pmid: 25672249 |
| [4] |
Li R.; Xiang K.; Peng Z. K.; Zou Y. Q.; Wang S. Y. Adv. Energy Mater. 2021, 11, 2102292.
doi: 10.1002/aenm.v11.46 |
| [5] |
Wang W. B.; Wen Q. L.; Liu Y. W.; Zhai T. Y. Acta Chim. Sinica 2020, 78, 1185. (in Chinese)
doi: 10.6023/A20060265 |
|
( 王文彬, 温群磊, 刘友文, 翟天佑, 化学学报, 2020, 78, 1185.)
doi: 10.6023/A20060265 |
|
| [6] |
Wang Z. H.; Ma C.; Fang P.; Xu H. C.; Mei T. S. Acta Chim. Sinica 2022, 80, 1115. (in Chinese)
doi: 10.6023/A22060260 |
|
( 王振华, 马聪, 方萍, 徐海超, 梅天胜, 化学学报, 2022, 80, 1115.)
doi: 10.6023/A22060260 |
|
| [7] |
Wang W. B.; Zhu Y. B.; Wen Q. L.; Wang Y. T.; Xia J.; Li C. C.; Chen M. W.; Liu Y. W.; Li H. Q.; Wu H. A.; Zhai T. Y. Adv. Mater. 2019, 31, 1900582.
doi: 10.1002/adma.v31.16 |
| [8] |
Si D.; Xiang B. Y.; Chen L. S.; Shi J. L. Chem Catal. 2021, 1, 941.
|
| [9] |
Wu K. H.; Zhou Y. W.; Ma X. Y.; Ding C.; Cai W. B. Acta Chim. Sinica 2018, 76, 292. (in Chinese)
doi: 10.6023/A17110478 |
|
( 吴匡衡, 周亚威, 马宪印, 丁辰, 蔡文斌, 化学学报, 2018, 76, 292.)
doi: 10.6023/A17110478 |
|
| [10] |
Xin L.; Zhang Z. Y.; Qi J.; Chadderdon D.; Li W. Z. Appl. Catal. B Environ. 2012, 125, 85.
doi: 10.1016/j.apcatb.2012.05.024 |
| [11] |
Zhang N. N.; Zou Y. Q.; Tao L.; Chen W.; Zhou L.; Liu Z. J.; Zhou B.; Huang G.; Lin H. Z.; Wang S. Y. Angew. Chem., Int. Ed. 2019, 58, 15895.
doi: 10.1002/anie.v58.44 |
| [12] |
Xu Z. H.; Rao L. X.; Song H. Y.; Yan Z. X.; Zhang L. J.; Yang S. B. Chin. J. Catal. 2017, 38, 305.
doi: 10.1016/S1872-2067(16)62560-3 |
| [13] |
Chen Z. D.; Chen Z. X.; Tang J. L.; Xu J.; Wang W. C.; Cao J. Y. Acta Chim. Sinica 2012, 70, 241. (in Chinese)
doi: 10.6023/A1106231 |
|
( 陈智栋, 陈转霞, 汤佳丽, 许娟, 王文昌, 曹剑瑜, 化学学报, 2012, 70, 241.)
doi: 10.6023/A1106231 |
|
| [14] |
Qin Y. C.; Zhang W. L.; Wang F. Q.; Li J. J.; Ye J. Y.; Sheng X.; Li C. X.; Liang X. Y.; Liu P.; Wang X. P.; Zheng X.; Ren Y. L.; Xu C. L.; Zhang Z. C. Angew. Chem., Int. Ed. 2022, 134, e202200899.
doi: 10.1002/ange.v134.16 |
| [15] |
Yan H.; Yao S.; Wang J. Y.; Zhao S. M.; Sun Y. H.; Liu M. Y.; Zhou X.; Zhang G. Y.; Jin X.; Feng X.; Liu Y. B.; Chen X. B.; Chen D.; Yang C. H. Appl. Catal., B 2021, 284, 119803.
doi: 10.1016/j.apcatb.2020.119803 |
| [16] |
Wang W. B.; Wang Y. T.; Yang R. O.; Wen Q. L.; Liu Y. W.; Jiang Z.; Li H. Q.; Zhai T. Y. Angew. Chem., Int. Ed. 2020, 59, 16974.
doi: 10.1002/anie.v59.39 |
| [17] |
Wen Q. L.; Lin Y.; Yang Y.; Gao R. J.; Ouyang N. Q.; Ding D. F.; Liu Y. W.; Zhai T. Y. ACS Nano 2022, 16, 9572.
doi: 10.1021/acsnano.2c02838 |
| [18] |
Wen Q. L.; Wang S. Z.; Wang R. W.; Huang D. J.; Fang J. K.; Liu Y. W.; Zhai T. Y. Nano Res. 2023, 16, 2286.
doi: 10.1007/s12274-022-5163-z |
| [19] |
Yong W. L.; Yang X. P.; Hou C. M.; Li B. J.; Gao H. T.; Lin J. H.; Luo X. L. Appl. Catal., B 2019, 259, 118020.
doi: 10.1016/j.apcatb.2019.118020 |
| [1] | Shan Li, Junxin Lu, Jie Liu, Lvqi Jiang, Wenbin Yi. Electrochemical Synthesis of α-Fluoroalkylated Ketones using Sodium Fluoroalkylsulfinate [J]. Acta Chimica Sinica, 2024, 82(2): 110-114. |
| [2] | Zhongshu Xie, Zhongxin Xue, Ziwen Xu, Qian Li, Hongyu Wang, Wei-Shi Li. Conjugated Crosslinking Modification of Graphitic Carbon Nitrides and Its Effect on Visible Light-Driven Photocatalytic Hydrogen Production [J]. Acta Chimica Sinica, 2022, 80(9): 1231-1237. |
| [3] | Shaobing Yan, Long Jiao, Chuanxin He, Hailong Jiang. Pyrolysis of ZIF-67/Graphene Composite to Co Nanoparticles Confined in N-Doped Carbon for Efficient Electrocatalytic Oxygen Reduction [J]. Acta Chimica Sinica, 2022, 80(8): 1084-1090. |
| [4] | Jiawei He, Liu Jiao, Xueyi Cheng, Guanghai Chen, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Structural Regulation of Metal Organic Framework-derived Hollow Carbon Nanocages and Their Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2022, 80(7): 896-902. |
| [5] | Yinlong Jiang, Guochao Li, Qingsong Chen, Zhongning Xu, Shanshan Lin, Guocong Guo. Porous Bismuth Nanoflowers Enriched with Lattice Dislocations for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Formate※ [J]. Acta Chimica Sinica, 2022, 80(6): 703-707. |
| [6] | Dan Wang, Bo Feng, Xiaoxin Zhang, Yanan Liu, Yan Pei, Minghua Qiao, Baoning Zong. Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide [J]. Acta Chimica Sinica, 2022, 80(6): 772-780. |
| [7] | Yu Qi, Fuxiang Zhang. Photocatalytic Water Splitting for Hydrogen Production※ [J]. Acta Chimica Sinica, 2022, 80(6): 827-838. |
| [8] | Pan An, Qinghui Zhang, Zhuang Yang, Jiaxing Wu, Jiaying Zhang, Yajun Wang, Yuming Li, Guiyuan Jiang. Research Progress of Solar Hydrogen Production Technology under Double Carbon Target [J]. Acta Chimica Sinica, 2022, 80(12): 1629-1642. |
| [9] | Jinge Wang, Wei Zhou, Jiayi Li, Yani Ding, Jihui Gao. Recent Advances and Performance Enhancement Mechanisms of Pulsed Electrocatalysis [J]. Acta Chimica Sinica, 2022, 80(11): 1555-1568. |
| [10] | Lili Cai, Jingyi Wang, Xuefeng Zhu, Weishen Yang. Recent Progress on Mixed Conducting Oxygen Transport Membrane Reactors for Water Splitting Reaction [J]. Acta Chimica Sinica, 2021, 79(5): 588-599. |
| [11] | Yining Ma, Run Shi, Tierui Zhang. Research Progress on Triphase Interface Electrocatalytic Carbon Dioxide Reduction [J]. Acta Chimica Sinica, 2021, 79(4): 369-377. |
| [12] | Su Zhan, Fuxiang Zhang. Recent Progress on Electrocatalytic Synthesis of Ammonia Under Amibent Conditions [J]. Acta Chimica Sinica, 2021, 79(2): 146-157. |
| [13] | Wu Qianye, Zhang Chenxi, Sun Kang, Jiang Hai-Long. Microwave-Assisted Synthesis and Photocatalytic Performance of a Soluble Porphyrinic MOF [J]. Acta Chimica Sinica, 2020, 78(7): 688-694. |
| [14] | Peng Zhengkang, Ding Huimin, Chen Rufan, Gao Chao, Wang Cheng. Research Progress in Covalent Organic Frameworks for Energy Storage and Conversion [J]. Acta Chim. Sinica, 2019, 77(8): 681-689. |
| [15] | Liu Shengwei, Zhao Jianjun, Xu Yiming. Larger Adsorption Effect of Fluoride than Phosphate on Phenol Degradation over the Irradiated Anatase TiO2 and Pt/TiO2 [J]. Acta Chim. Sinica, 2019, 77(4): 351-357. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||