Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (10): 1039-1049.DOI: 10.6023/A24050152 Previous Articles Next Articles
Article
税子怡a,b, 于思乐c, 陆伟a, 许留云b, 刘庆叶a, 赵炜a,*( ), 刘益伦d
), 刘益伦d
投稿日期:2024-05-06
发布日期:2024-07-09
基金资助:
Ziyi Shuia,b, Sile Yuc, Wei Lua, Liuyun Xub, Qingye Liua, Wei Zhaoa( ), Yilun Liud
), Yilun Liud
Received:2024-05-06
Published:2024-07-09
Contact:
*E-mail: zhaowei3313@nwu.edu.cn
Supported by:Share
Ziyi Shui, Sile Yu, Wei Lu, Liuyun Xu, Qingye Liu, Wei Zhao, Yilun Liu. Bifunctional Electrocatalysts of Mn-doped Co3O4 for Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Medium[J]. Acta Chimica Sinica, 2024, 82(10): 1039-1049.
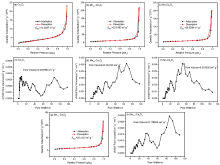

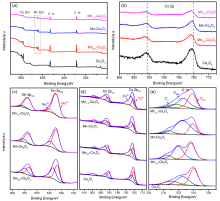

| 样品 | 元素 | XPS结果 | ICP结果 | EDS结果 | ||||
|---|---|---|---|---|---|---|---|---|
| 原子比(%) | Mn/Co | 原子比(%) | Mn/Co | 原子比(%) | Mn/Co | |||
| Co3O4 | Mn | 0 | 0 | 0 | 0 | 0 | 0 | |
| Co | 8.64 | 69.65 | 46.55 | |||||
| Mn0.5-Co3O4 | Mn | 3.28 | 0.53 | 25.93 | 0.49 | 18.60 | 0.51 | |
| Co | 6.21 | 51.99 | 36.14 | |||||
| Mn-Co3O4 | Mn | 4.51 | 0.82 | 36.31 | 0.77 | 25.4 | 0.83 | |
| Co | 5.48 | 46.80 | 30.5 | |||||
| Mn1.5-Co3O4 | Mn | 5.12 | 1.21 | 44.94 | 1.19 | 30.07 | 1.21 | |
| Co | 4.23 | 37.55 | 24.84 | |||||
| 样品 | 元素 | XPS结果 | ICP结果 | EDS结果 | ||||
|---|---|---|---|---|---|---|---|---|
| 原子比(%) | Mn/Co | 原子比(%) | Mn/Co | 原子比(%) | Mn/Co | |||
| Co3O4 | Mn | 0 | 0 | 0 | 0 | 0 | 0 | |
| Co | 8.64 | 69.65 | 46.55 | |||||
| Mn0.5-Co3O4 | Mn | 3.28 | 0.53 | 25.93 | 0.49 | 18.60 | 0.51 | |
| Co | 6.21 | 51.99 | 36.14 | |||||
| Mn-Co3O4 | Mn | 4.51 | 0.82 | 36.31 | 0.77 | 25.4 | 0.83 | |
| Co | 5.48 | 46.80 | 30.5 | |||||
| Mn1.5-Co3O4 | Mn | 5.12 | 1.21 | 44.94 | 1.19 | 30.07 | 1.21 | |
| Co | 4.23 | 37.55 | 24.84 | |||||
| Oads/Olatt | Mn3+/Mn4+ | Co2+/Co3+ | Co3+/Mn3+ | |
|---|---|---|---|---|
| Co3O4 | 0.68 | — | 0.88 | |
| Mn0.5-Co3O4 | 0.98 | 1.37 | 0.94 | 1.13 |
| Mn-Co3O4 | 1.76 | 1.98 | 1.25 | 0.91 |
| Mn1.5-Co3O4 | 0.90 | 2.23 | 1.18 | 0.32 |
| Oads/Olatt | Mn3+/Mn4+ | Co2+/Co3+ | Co3+/Mn3+ | |
|---|---|---|---|---|
| Co3O4 | 0.68 | — | 0.88 | |
| Mn0.5-Co3O4 | 0.98 | 1.37 | 0.94 | 1.13 |
| Mn-Co3O4 | 1.76 | 1.98 | 1.25 | 0.91 |
| Mn1.5-Co3O4 | 0.90 | 2.23 | 1.18 | 0.32 |




| Co3O4 | Mn0.5-Co3O4 | Mn-Co3O4 | Mn1.5-Co3O4 | ||
|---|---|---|---|---|---|
| ORR | 起始电位/V | 0.75 | 0.83 | 0.85 | 0.78 |
| 半波电位/V | 0.65 | 0.66 | 0.69 | 0.58 | |
| Tafel斜率/ (mV•dec−1) | 134.4 | 132.9 | 103.7 | 197.9 | |
| 电子转移数 | 3.15 | 3.36 | 3.57 | 3.29 | |
| OER | Ej=10/V | 1.791 | 1.789 | 1.781 | 1.805 |
| 相应过电位/ mV | 0.561 | 0.559 | 0.551 | 0.575 | |
| Tafel斜率/ (mV•dec−1) | 91.3 | 99.2 | 88.1 | 98.3 |
| Co3O4 | Mn0.5-Co3O4 | Mn-Co3O4 | Mn1.5-Co3O4 | ||
|---|---|---|---|---|---|
| ORR | 起始电位/V | 0.75 | 0.83 | 0.85 | 0.78 |
| 半波电位/V | 0.65 | 0.66 | 0.69 | 0.58 | |
| Tafel斜率/ (mV•dec−1) | 134.4 | 132.9 | 103.7 | 197.9 | |
| 电子转移数 | 3.15 | 3.36 | 3.57 | 3.29 | |
| OER | Ej=10/V | 1.791 | 1.789 | 1.781 | 1.805 |
| 相应过电位/ mV | 0.561 | 0.559 | 0.551 | 0.575 | |
| Tafel斜率/ (mV•dec−1) | 91.3 | 99.2 | 88.1 | 98.3 |
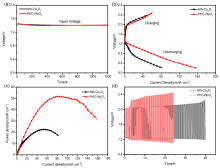

| [1] |
Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Electrochim. Acta 2019, 300 455.
doi: 10.1016/j.electacta.2019.01.092 |
| [2] |
Miao, H.; Wu, X.; Chen, B.; Wang, Q.; Wang, F.; Wang, J.; Zhang, C.; Zhang, H.; Yuan, J.; Zhang, Q. Electrochim. Acta 2020, 333 135566.
|
| [3] |
Dai, Y.; Yu, J.; Cheng, C.; Tan, P.; Ni, M. Chem. Eng. J. 2020, 397 125516.
|
| [4] |
Lankauf, K.; Cysewska, K.; Karczewski, J.; Mielewczyk-Gryń, A.; Górnicka, K.; Cempura, G.; Chen, M.; Jasiński, P.; Molin, S. Int. J. Hydrogen Energy 2020, 45 14867.
|
| [5] |
Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. Appl. Surf. Sci. 2021, 536 147806.
|
| [6] |
Béjar, J.; Álvarez-Contreras, L.; Ledesma-García, J.; Arjona, N.; Arriaga, L. G. J. Electroanal. Chem. 2019, 847 113190.
|
| [7] |
Wang, Y.; Zhang, L.; Hu, T. Acta Chim. Sinica 2015, 73 316 (in Chinese).
doi: 10.6023/A14110787 |
|
(王瀛, 张丽敏, 胡天军, 化学学报, 2015, 73 316.)
doi: 10.6023/A14110787 |
|
| [8] |
Liu, H.; Zhu, X.; Li, M.; Tang, Q.; Sun, G.; Yang, W. Electrochim. Acta 2014, 144 31.
|
| [9] |
Liu, J.; Yang, N.; Li, L.; Wei, Z. Acta Chim. Sinica 2023, 81 1478 (in Chinese).
|
|
(刘金晶, 杨娜, 李莉, 魏子栋, 化学学报, 2023, 81 1478.)
doi: 10.6023/A23050221 |
|
| [10] |
Shi, C.; Ullah, S.; Li, K.; Wang, W.; Zhang, R.; Pan, L.; Zhang, X.; Zou, J.-J. Chinese J. Catal. 2020, 41 1818.
|
| [11] |
Chang, J.; Wang, W.; Wang, Y.; Su, C.; Pan, J.; Wang, H.; Song, H. J. Taiwan Inst. Chem. Eng. 2021, 129 144.
|
| [12] |
Fujiwara, N.; Nagai, T.; Ioroi, T.; Arai, H.; Ogumi, Z. J. Power Sources 2020, 451 227736.
|
| [13] |
Hu, S.; Wang, J.; Zhang, J.; Lim, J.; Gao, Y.; Zhang, S. Appl. Catal. B: Environ. 2021, 282 119593.
|
| [14] |
Sun, Y.-R.; Zhang, X.; Wang, L.-G.; Liu, Z.-K.; Kang, N.; Zhou, N.; You, W.-L.; Li, J.; Yu, X.-F. Chem. Eng. J. 2021, 421 129698.
|
| [15] |
Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Nat. Mater. 2011, 10 780.
|
| [16] |
Song, K.; Yuan, L.; Li, Z.; Lv, Y.; Yang, B.; Yu, Y.; Shen, X.; Hu, X. Electrochim. Acta 2020, 353 136572.
|
| [17] |
Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M. T.; Croft, M.; Gopalakrishnan, J.; Pena, M. A.; Hadermann, J.; Greenblatt, M.; Rojas, S. ACS Appl. Mater. Interfaces 2019, 11 21454.
|
| [18] |
Jung, J.-I.; Risch, M.; Park, S.; Kim, M. G.; Nam, G.; Jeong, H.-Y.; Shao-Horn, Y.; Cho, J. Energy & Environ. Sci. 2016, 9 176.
|
| [19] |
Kim, J.; Ko, W.; Yoo, J. M.; Paidi, V. K.; Jang, H. Y.; Shepit, M.; Lee, J.; Chang, H.; Lee, H. S.; Jo, J.; Kim, B. H.; Cho, S. P.; Lierop, J.; Kim, D.; Lee, K. S.; Back, S.; Sung, Y. E.; Hyeon, T. Adv. Mater. 2022, 34, e2107868.
|
| [20] |
Maile, N. C.; Moztahida, M.; Ghani, A. A.; Hussain, M.; Tahir, K.; Kim, B.; Shinde, S. K.; Fulari, V. J.; Lee, D. S. Chem. Eng. J. 2021, 421 129767.
|
| [21] |
Shui, Z.; He, N.; Chen, L.; Zhao, W.; Chen, X. Acta Chim. Sinica 2020, 78 557 (in Chinese).
doi: 10.6023/A20030068 |
|
(税子怡, 何娜娜, 陈黎, 赵炜, 陈曦, 化学学报, 2020, 78 557.)
doi: 10.6023/A20030068 |
|
| [22] |
Li, K.; Zhang, R.; Gao, R.; Shen, G.-Q.; Pan, L.; Yao, Y.; Yu, K.; Zhang, X.; Zou, J.-J. Appl. Catal. B: Environ. 2019, 244 536.
|
| [23] |
Li, Z.; Lv, Y.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. J. Alloys Compd. 2020, 817 152736.
|
| [24] |
Ge, C.; Li, Q.; Hu, M.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. J. Power Sources 2022, 520 230868.
|
| [25] |
Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. J. Am. Chem. Soc. 2012, 134 3517.
|
| [26] |
Bae, J.; Shin, D.; Jeong, H.; Choe, C.; Choi, Y.; Han, J. W.; Lee, H. ACS Catal. 2021, 11 11066.
|
| [27] |
Zhu, F.; Zhang, J.; Yang, B.; Shi, X.; Lu, C.; Yin, J.; Yu, Y.; Hu, X. J. Alloys Compd. 2018, 749 433.
|
| [28] |
Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Nat. Commun. 2015, 6 7345.
|
| [29] |
Zhang, W.; Li, M.; Wang, X.; Zhang, X.; Niu, X.; Zhu, Y. Appl. Surf. Sci. 2022, 590 153140.
|
| [30] |
Qin, C.; Wang, B.; Wang, Y. Sens. Actuators B: Chem. 2022, 351 130943.
|
| [31] |
Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Zhan, F. J. Power Sources 2015, 299 492.
|
| [32] |
Cheng, H.; Chen, J. M.; Li, Q. J.; Su, C. Y.; Chen, A. N.; Zhang, J. X.; Liu, Z. Q.; Tong, Y. Chem. Commun. 2017, 53 11596.
|
| [33] |
Bae, J.; Shin, D.; Jeong, H.; Kim, B.-S.; Han, J. W.; Lee, H. ACS Catal. 2019, 9 10093.
|
| [34] |
Wu, J.; Wang, X.; Zheng, W.; Sun, Y.; Xie, Y.; Ma, K.; Zhang, Z.; Liao, Q.; Tian, Z.; Kang, Z.; Zhang, Y. J. Am. Chem. Soc. 2022, 144 19163.
|
| [35] |
Sun, Y.; Wu, J.; Xie, Y.; Wang, X.; Ma, K.; Tian, Z.; Zhang, Z.; Liao, Q.; Zheng, W.; Kang, Z.; Zhang, Y. Adv. Funct. Mater. 2022, 32 2207116.
|
| [36] |
Tyagi, A.; Penke, Y. K.; Sinha, P.; Malik, I.; Kar, K. K.; Ramkumar, J.; Yokoi, H. Int. J. Hydrogen Energy 2021, 46 22434.
|
| [37] |
Xu, W.; Apodaca, N.; Wang, H.; Yan, L.; Chen, G.; Zhou, M.; Ding, D.; Choudhury, P.; Luo, H. ACS Catal. 2019, 9 5074.
|
| [38] |
Shi, Y.; Liu, T.; Zhao, Y.; Su, J.; Zeb, S.; Nie, Y.; Qin, C.; Wang, B.; Jiang, X. Sens. Actuators B: Chem. 2022, 350 130860.
|
| [39] |
Ho, J.; Li, Y.; Dai, Y.; Kim, T.; Wang, J.; Ren, J.; Yun, H.; Liu, X. Int. J. Hydrogen Energy 2021, 46 20503.
|
| [40] |
Hu, Z.; Zhou, X.; Lu, Y.; Jv, R.; Liu, Y.; Li, N.; Chen, S. Electrochim. Acta 2019, 296 214.
|
| [41] |
Zou, J.; Chen, B.; Li, B.; Yin, M.; Miao, H.; Wang, F.; Zhang, C.; Zhang, H.; Yuan, J. Int. J. Hydrogen Energy 2022, 47 27470.
|
| [1] | Fanfan Zhang, Yuantao Cai, Jianbo Tao, Guoju Chang, Xinchen Guo, Shiyou Hao. Effect of Zn, C Introduction Amount and Calcination Temperature on the Photocatalytic Reduction of Cu2+over ZnO/C/CeO2 [J]. Acta Chimica Sinica, 2024, 82(8): 871-878. |
| [2] | Guojing Wang, Yonghui Chen, Xiuqin Zhang, Junsheng Zhang, Junmin Xu, Jing Wang. Magnetic and Photoelectrocatalytic Properties of BiVO4 Surface Heterojunctions Controlled by Oxygen Vacancies [J]. Acta Chimica Sinica, 2024, 82(4): 409-415. |
| [3] | Ping Li, Qiyu Yang, Jing Zeng, Ran Zhang, Qiuyan Chen, Fei Yan. Effect of Fluorine Doping on the Performance of Reversible Solid Oxide Cells and Related Kinetic Studies [J]. Acta Chimica Sinica, 2024, 82(1): 36-45. |
| [4] | Yuan Zhang, Beining Zheng, Meichun Fu, Shouhua Feng. Research Progress in the Application of Spinel Oxides in Tumor Therapy★ [J]. Acta Chimica Sinica, 2023, 81(8): 949-954. |
| [5] | Jinjing Liu, Na Yang, Li Li, Zidong Wei. Theoretical Study on the Regulation of Oxygen Reduction Mechanism by Modulating the Spatial Structure of Active Sites on Platinum★ [J]. Acta Chimica Sinica, 2023, 81(11): 1478-1485. |
| [6] | Shaobing Yan, Long Jiao, Chuanxin He, Hailong Jiang. Pyrolysis of ZIF-67/Graphene Composite to Co Nanoparticles Confined in N-Doped Carbon for Efficient Electrocatalytic Oxygen Reduction [J]. Acta Chimica Sinica, 2022, 80(8): 1084-1090. |
| [7] | Dan Wang, Bo Feng, Xiaoxin Zhang, Yanan Liu, Yan Pei, Minghua Qiao, Baoning Zong. Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide [J]. Acta Chimica Sinica, 2022, 80(6): 772-780. |
| [8] | Yuan Lu, Jifen Wang, Huaqing Xie. First-principles Study on Low Index Surface Structure Optimization and Surface Energy of LiMn2O4 Spinel Oxides [J]. Acta Chimica Sinica, 2021, 79(8): 1058-1064. |
| [9] | Si Wang, Jialing Ma, Lifang Chen, Xin Zhang. Role of Synergistic Effect in Oxygen Evolution Reaction over Layered Double Hydroxide [J]. Acta Chimica Sinica, 2021, 79(2): 216-222. |
| [10] | Lu Xiaoqing, Cao Shoufu, Wei Xiaofei, Li Shaoren, Wei Shuxian. Investigation on Oxygen Reduction Reaction Mechanism on S Doped Fe-NC lsolated Single Atoms Catalyst [J]. Acta Chimica Sinica, 2020, 78(9): 1001-1006. |
| [11] | Shui Ziyi, He Nana, Chen Li, Zhao Wei, Chen Xi. Porous Perovskite towards Oxygen Reduction Reaction in Flexible Aluminum-Air Battery [J]. Acta Chimica Sinica, 2020, 78(6): 557-564. |
| [12] | Dai Mimi, Wang Jian, Li Linge, Wang Qi, Liu Meinan, Zhang Yuegang. High-performance Oxygen Evolution Catalyst Enabled by Interfacial Effect between CeO2 and FeNi Metal-organic Framework [J]. Acta Chimica Sinica, 2020, 78(4): 355-362. |
| [13] | Zhang Xuhan, Deng Bowen, Fan Haidong, Huang Wenhui, Zhang Yanwei. Photo-thermochemical CO2 Splitting Based on Zinc-germanium Binary Oxide [J]. Acta Chimica Sinica, 2020, 78(10): 1120-1126. |
| [14] | Wang Yilin, Wang Minjie, Li Jing, Wei Zidong. Iron/nickel Alloy Nanoparticles Embedded in N-doped Porous Carbon for Robust Oxygen Evolution Reaction [J]. Acta Chim. Sinica, 2019, 77(1): 84-89. |
| [15] | Huang Wenjiao, Zhang Haoyu, Hu Shuozhen, Niu Dongfang, Zhang Xinsheng. Effect of Nitrogen-Containing Functional Groups of Cobalt Phthalocyanine Catalyst on the Oxygen Reduction Performance in Fuel Cells [J]. Acta Chim. Sinica, 2018, 76(9): 723-728. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||