Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (8): 871-878.DOI: 10.6023/A24040144 Previous Articles Next Articles
Article
张帆帆†, 蔡元韬†, 陶剑波†, 常国菊, 郭欣辰, 郝仕油*( )
)
投稿日期:2024-04-25
发布日期:2024-07-22
作者简介:基金资助:
Fanfan Zhang†, Yuantao Cai†, Jianbo Tao†, Guoju Chang, Xinchen Guo, Shiyou Hao* )
)
Received:2024-04-25
Published:2024-07-22
Contact:
* E-mail: About author:Supported by:Share
Fanfan Zhang, Yuantao Cai, Jianbo Tao, Guoju Chang, Xinchen Guo, Shiyou Hao. Effect of Zn, C Introduction Amount and Calcination Temperature on the Photocatalytic Reduction of Cu2+over ZnO/C/CeO2[J]. Acta Chimica Sinica, 2024, 82(8): 871-878.


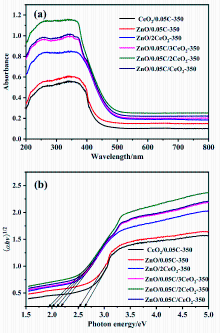
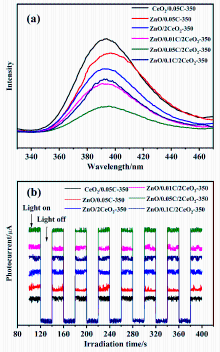
| Sample | Area of C=C | Area of C=O | Area of O—C=O | Total Area |
|---|---|---|---|---|
| ZnO/0.05C/2CeO2-350 | 4882.488 | 825.3487 | 1244.586 | 6952.4227 |
| ZnO/0.05C/2CeO2-450 | 4496.099 | 401.5865 | 805.8344 | 5703.5199 |
| ZnO/0.05C/2CeO2-550 | 3459.989 | 893.3685 | 1177.464 | 5530.8215 |
| Sample | Area of C=C | Area of C=O | Area of O—C=O | Total Area |
|---|---|---|---|---|
| ZnO/0.05C/2CeO2-350 | 4882.488 | 825.3487 | 1244.586 | 6952.4227 |
| ZnO/0.05C/2CeO2-450 | 4496.099 | 401.5865 | 805.8344 | 5703.5199 |
| ZnO/0.05C/2CeO2-550 | 3459.989 | 893.3685 | 1177.464 | 5530.8215 |
| [1] |
Fu, F.; Wang, Q. J. Environ. Manage. 2011, 92, 407.
|
| [2] |
Aziz, H. A.; Adlan, M. N.; Ariffin, K. S. Biores. Technol. 2008, 99, 1578.
|
| [3] |
Huang, Z.; Lu, L.; Cai, Z. X.; Ren, Z. J. J. Hazard. Mater. 2016, 302, 323.
doi: S0304-3894(15)30119-9 pmid: 26476320 |
| [4] |
Ihsanullah.; Abbas, A.; Al-Amer, A. M.; Laoui, T.; Al-Marri, M. J.; Nasser, M. S.; Khraisheh, M.; Atieh, M. A. Sep. Purif. Technol. 2016, 157, 141.
|
| [5] |
Dong, C. C.; Lu, J.; Qiu, B. C.; Shen, B.; Xing, M. Y.; Zhang, J. L. Appl. Catal., B 2018, 222, 146.
|
| [6] |
Xu, Z.; Shan, C.; Xie, B. H.; Liu, Y.; Pan, B. C. Appl. Catal., B 2017, 200, 439.
|
| [7] |
Joseph, H. M.; Poornima, N. Mater. Today 2019, 9, 7.
|
| [8] |
Zhong, W.; Xia, Y. F.; Zhai, H. L.; Gao, Y.; Li, S. H.; Lv, C. X. Chinese J. Inorg. Chem. 2020, 36, 40 (in Chinese).
|
|
(钟伟, 夏颖帆, 翟杭玲, 高越, 李世慧, 吕春欣, 无机化学学报, 2020, 36, 40.)
|
|
| [9] |
Umar, A.; Kumar, R.; Akhtar, M. S.; Kumar, G.; Kim, S. H. J. Colloid Interf. Sci. 2015, 454, 61.
|
| [10] |
Li, C. Q.; Luo, L. T.; Xiong, G. W. Acta Chim. Sinica 2010, 68, 1023 (in Chinese).
|
|
(李长全, 罗来涛, 熊光伟, 化学学报, 2010, 68, 1023.)
|
|
| [11] |
Pathak, V.; Lad, P.; Thakkar, A. B.; Thakor, P.; Deshpande, M. P.; Pandya, S. Inorg. Chem. Commun. 2024, 159, 111738.
|
| [12] |
Sun, X. C.; Yuan, K.; Hua, W. D.; Gao, Z. R.; Zhang, Q.; Yuan, C. Y.; Liu, H. C.; Zhang, Y. W. ACS Catal. 2022, 12, 11942.
|
| [13] |
Celebi, N.; Salimi, K. J. Colloid Interf. Sci. 2022, 605, 23.
|
| [14] |
Yu, L. X.; Yang, L. Y.; Chen, H. S.; Tao, J. B.; Hao, S. Y. J. Rare Earths 2021, 39, 238 (in Chinese).
|
|
(余丽霞, 杨丽媛, 陈寒松, 陶剑波, 郝仕油, 中国稀土学报, 2021, 39, 238.)
|
|
| [15] |
Wang, H.; Shang, J.; Xiao, Z. L.; Aprea, P.; Hao, S. Y. Dyes Pigm. 2020, 182, 108669.
|
| [16] |
Sun, Q. P.; He, Z. W.; Tan, H. Y.; Aprea, P.; Hao, S. Y. Ceram. Int. 2022, 48, 26846.
|
| [17] |
Ahmad, N.; Umar, A.; Kumar, R.; Alam, M. Ceram. Int. 2016, 42, 11562.
|
| [18] |
Manikanika, L.; Chopra, L.; Kumar, R. Inorg. Chem. Commun. 2024, 160, 111896.
|
| [19] |
Chen, Y.; Zhang, L.; Liu, Z.; Qiu, K. H. Chem. Eng. Technol. 2017, 7, 11 (in Chinese).
|
|
(陈越, 张力, 刘铸, 邱克辉, 化学工程与技术, 2017, 7, 11.)
|
|
| [20] |
Imtiaz, A.; Farrukh, M. A.; Latif, H. J. Mol. Struct. 2024, 1300, 137174.
|
| [21] |
Xu, D. Y.; Cheng, F.; Lu, Q. Z.; Dai, P. Ind. Eng. Chem. Res. 2014, 53, 2625.
|
| [22] |
Sabzehmeidani, M. M.; Karimi, H.; Ghaedi, M. New J. Chem. 2020, 44, 5033.
|
| [23] |
Yue, L.; Zhang, X. M. J. Alloys Compd. 2009, 475, 702.
|
| [24] |
Wang, X. Q.; Rodriguez, J. A.; Hanson, J. C.; Gamarra, D.; Martinez-Arias, A.; Fernandez-Garcia, M. J. Phys. Chem. B 2005, 109, 19595.
|
| [25] |
Albrecht, P. M.; Jiang, D. E.; Mullins, D. R. J. Phys. Chem. C 2014, 118, 9042.
|
| [26] |
Zhang, P.; Zhang, H. W.; Wang, S. H.; Lei, X. Q.; Yang, J. T.; Li, Z. M.; Zhu, H. B.; Bao, X. J.; Yuan, P. J. Mater. Sci. 2020, 55, 12876.
|
| [27] |
Jiang, H. Y.; Liu, G. G.; Li, M.; Liu, J. J.; Sun, W. B.; Ye, J. H.; Lin, J. Appl. Catal., B 2015, 163, 267.
|
| [28] |
Chen, S. B.; Li, X.; Zhou, W. Y.; Zhang, S. S.; Fang, Y. P. Appl. Surf. Sci. 2018, 466, 254.
|
| [29] |
Lei, W. J.; Wang, H.; Zhang, X. J.; Yang, Z. M.; Kong, C. C. Ceram. Int. 2022, 48, 1757.
|
| [30] |
Zhang, X.; Matras-Postolek, K.; Yang, P.; Jiang, S. P. J. Colloid Interf. Sci. 2023, 636, 646.
doi: 10.1016/j.jcis.2023.01.052 pmid: 36680955 |
| [31] |
Xue, Y. F.; Tian, D.; Zhang, D. X.; Zeng, C. H.; Fu, Y. C.; Li, K. Z.; Wang, H.; Tian, Y. F. Comput. Mater. Sci. 2019, 158, 197.
|
| [32] |
Ma, D. D.; Sun, D. K.; Zou, Y. J.; Mao, S. M.; Lv, Y. X.; Wang, Y.; Li, J.; Shi, J. W. J. Colloid Interf. Sci. 2019, 549, 179.
|
| [33] |
Chen, M. Y.; Zu, X. T.; Xiang, X.; Zhang, H. L. Physica B 2006, 389, 263.
|
| [34] |
Laguna, O. H.; Centeno, M. A.; Boutonnet, M.; Odriozola, J. A. Appl. Catal., B 2011, 106, 621.
|
| [35] |
Xu, J.; Li, M.; Qiu, J. H.; Zhang, X. F.; Feng, Y.; Yao, J. F. Chem. Eng. J. 2020, 383, 123135.
|
| [36] |
Zangeneh, H.; Farhadian, M.; Zinatizadeh, A. A. J. Environ. Chem. Eng. 2020, 8, 103639.
|
| [37] |
Vignesh, S.; Suganthi, S.; Sundar, J. K.; Raj, V. Appl. Surf. Sci. 2019, 488, 763.
|
| [38] |
Zheng, X. G.; Chen, Q.; Lv, S. H.; Fu, X. J.; Wen, J.; Liu, X. H. Nanomaterials 2019, 9, 1643.
|
| [39] |
Wen, W. J.; Lou, Z. C.; Chen, Y. J.; Chen, D. S.; Tian, S. H.; Xiong, Y. J. Chem. Technol. Biotechnol. 2019, 94, 1576.
|
| [40] |
Tan, H. Y.; Xiao, Z. L.; Jiang, S. J.; Sun, Q. P.; Hao, S. Y. Acta Scien. Circum. 2023, 43, 77 (in Chinese).
|
|
(谭贺云, 肖忠连, 蒋森杰, 孙巧萍, 郝仕油, 环境科学学报, 2023, 43, 77.)
|
| [1] | Guojing Wang, Yonghui Chen, Xiuqin Zhang, Junsheng Zhang, Junmin Xu, Jing Wang. Magnetic and Photoelectrocatalytic Properties of BiVO4 Surface Heterojunctions Controlled by Oxygen Vacancies [J]. Acta Chimica Sinica, 2024, 82(4): 409-415. |
| [2] | Zhang Xuhan, Deng Bowen, Fan Haidong, Huang Wenhui, Zhang Yanwei. Photo-thermochemical CO2 Splitting Based on Zinc-germanium Binary Oxide [J]. Acta Chimica Sinica, 2020, 78(10): 1120-1126. |
| [3] | Xu Chenyu, Lin Jiayi, Pan Fuqiang, Deng Bowen, Wang Zhihua, Zhou Junhu, Chen Yun, Ma Jingcheng, Gu Zhien, Zhang Yanwei. Photo-thermochemical Cycle for CO2 Reduction based on Effective Ni ion Substitute-doped TiO2 [J]. Acta Chim. Sinica, 2017, 75(7): 699-707. |
| [4] | Liu Lilu, Qi Xingguo, Hu Yongsheng, Chen Liquan, Huang Xuejie. Novel Cu Based Oxides with Tunnel Structure as Cathode for Sodium-ion Batteries [J]. Acta Chim. Sinica, 2017, 75(2): 218-224. |
| [5] | Li Yuan, Liu Wenna, Zheng Xingwang. Investigation into Electrogenerated Chemiluminescence Behavior of Tris(bipyridine)ruthenium(II)/silica Nanoparticles Electrocatalyzed by Cu(tris(hydroxymethyl)aminomethane)42+ Complex [J]. Acta Chim. Sinica, 2015, 73(7): 749-754. |
| [6] | Gao Mengyu, Jiang Dong, Sun Dekui, Hou Bo, Li Debao. Synthesis of Ag/N-TiO2/SBA-15 Photocatalysts and Photocatalytic Reduction of CO2 under Visible Light Irradiation [J]. Acta Chimica Sinica, 2014, 72(10): 1092-1098. |
| [7] | Wang Huixiang, Jiang Dong, Wu Dong, Li Debao, Sun Yuhan. Synthesis of Supported TiO2/SBA-15 Catalysts and Their Performance on Photocatalytic Reduction of CO2 [J]. Acta Chimica Sinica, 2012, 70(23): 2412-2418. |
| [8] | Zhang Hua, Liu Aihong, Wu Fangying. Synthesis of s-Triazine Derivatives and Using as Colorimetric Probe for Selective Recognizing of Fe3+ and Cu2+ [J]. Acta Chimica Sinica, 2012, 70(08): 1001-1007. |
| [9] | DU Hai-Long, ZHANG Yong, SHENG Min-Qi, LIU Wei-Dong, ZHONG Qing-Dong, WANG Yi, ZHOU Qiong-Yu. Study on Electrochemistry of Oxide Films Formed on the Surface of Cobalt-tungsten Alloy Coatings in Alkali Solution [J]. Acta Chimica Sinica, 2011, 69(08): 881-888. |
| [10] | HAO Lan WANG Yan* CHEN Guang-Ju*. Theoretical Study on the Adsorption of CO and O2 on MgO-Supported Pd and Cu Atoms [J]. Acta Chimica Sinica, 2008, 66(9): 1028-1036. |
| [11] | LIU Ya-Qin, XU Yao, LI Zhi-Jie, ZHANG Xiu-Ping, WU Dong, SUN Yu-Han*. Photocatalytic Reduction of CO2 in the Suspension System of Nano-SiO2/TiO2 [J]. Acta Chimica Sinica, 2006, 64(6): 453-457. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||