Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (4): 341-353.DOI: 10.6023/A25020034 Previous Articles Next Articles
Article
于千尧a, 孟铭a, 姚景方a, 杜姗姗b,*( ), 齐昀坤a,*(
), 齐昀坤a,*( )
)
投稿日期:2025-02-02
发布日期:2025-04-07
作者简介: † 共同第一作者.
基金资助:
Qianyao Yua, Ming Menga, Jingfang Yaoa, Shanshan Dub( ), Yunkun Qia(
), Yunkun Qia( )
)
Received:2025-02-02
Published:2025-04-07
Contact:
E-mail: About author: † These authors contributed equally to this work.
Supported by:Share
Qianyao Yu, Ming Meng, Jingfang Yao, Shanshan Du, Yunkun Qi. Two Step Synthesis of Octreotide-doxorubicin Conjugates Based on Disulfide Bond Linker[J]. Acta Chimica Sinica, 2025, 83(4): 341-353.

| Peptides | Resin | SPPS strategy | Peptide cleavage cocktails | Peptide cleavage time/h | RP-HPLC purity/% | Isolated yield/% | Yield/mg |
|---|---|---|---|---|---|---|---|
| 1 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/EDTa | 2 | 16.4 | 6.3 | 4.1 |
| 2 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/ DODTb | 2 | Not detected | Not detected | Not detected |
| 3 | Wang resin | SPPS-1 | TFA/TIPS/waterc | 2 | 23.4 | 8.3 | 5.4 |
| 4 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/EDT | 2 | 24.2 | 9.4 | 6.1 |
| 5 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/DODT | 2 | 61.3 | 18.5 | 12.0 |
| 6 | Wang resin | SPPS-2 | TFA/TIPS/water | 2 | 77.6 | 28.3 | 18.4 |
| 7 | Wang resin | SPPS-2 | TFA/TIPS/water | 3 | 71.3 | 21.6 | 14.0 |
| 8 | 2-Chlorotrityl chloride resin | SPPS-2 | TFA/TIPS/water | 2 | 74.6 | 24.4 | 15.8 |
| Peptides | Resin | SPPS strategy | Peptide cleavage cocktails | Peptide cleavage time/h | RP-HPLC purity/% | Isolated yield/% | Yield/mg |
|---|---|---|---|---|---|---|---|
| 1 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/EDTa | 2 | 16.4 | 6.3 | 4.1 |
| 2 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/ DODTb | 2 | Not detected | Not detected | Not detected |
| 3 | Wang resin | SPPS-1 | TFA/TIPS/waterc | 2 | 23.4 | 8.3 | 5.4 |
| 4 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/EDT | 2 | 24.2 | 9.4 | 6.1 |
| 5 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/DODT | 2 | 61.3 | 18.5 | 12.0 |
| 6 | Wang resin | SPPS-2 | TFA/TIPS/water | 2 | 77.6 | 28.3 | 18.4 |
| 7 | Wang resin | SPPS-2 | TFA/TIPS/water | 3 | 71.3 | 21.6 | 14.0 |
| 8 | 2-Chlorotrityl chloride resin | SPPS-2 | TFA/TIPS/water | 2 | 74.6 | 24.4 | 15.8 |



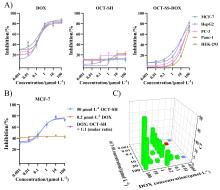

| Combined agents | Agents | MCF-7 | HepG2 | PC-3 | Panc-1 | HEK-293 | SIa | SIb |
|---|---|---|---|---|---|---|---|---|
| — | OCT-SH | >100 | >100 | >100 | >100 | >100 | — | — |
| — | OCT-SS-DOX | 12.56±1.23 | 13.26±2.11 | 27.64±2.35 | 28.82±3.44 | 48.79±3.98 | 3.88 | 1.77 |
| — | DOX | 0.25±0.02 | 0.32±0.06 | 0.26±0.04 | 0.36±0.08 | 0.15±0.04 | 0.60 | 0.58 |
| 50 μmol/L OCT-SH | DOX | 0.31±0.08 | — | — | — | — | — | — |
| 0.2 μmol/L DOX | OCT-SH | >100 | — | — | — | — | — | — |
| DOX/OCT-SH (1∶1, molar ratio) | 0.28±0.06 | — | — | — | — | — | — | |
| Combined agents | Agents | MCF-7 | HepG2 | PC-3 | Panc-1 | HEK-293 | SIa | SIb |
|---|---|---|---|---|---|---|---|---|
| — | OCT-SH | >100 | >100 | >100 | >100 | >100 | — | — |
| — | OCT-SS-DOX | 12.56±1.23 | 13.26±2.11 | 27.64±2.35 | 28.82±3.44 | 48.79±3.98 | 3.88 | 1.77 |
| — | DOX | 0.25±0.02 | 0.32±0.06 | 0.26±0.04 | 0.36±0.08 | 0.15±0.04 | 0.60 | 0.58 |
| 50 μmol/L OCT-SH | DOX | 0.31±0.08 | — | — | — | — | — | — |
| 0.2 μmol/L DOX | OCT-SH | >100 | — | — | — | — | — | — |
| DOX/OCT-SH (1∶1, molar ratio) | 0.28±0.06 | — | — | — | — | — | — | |

| [1] |
Siegel, R. L.; Giaquinto, A. N.; Jemal, A. CA-Cancer J. Clin. 2024, 74, 12.
|
| [2] |
Anderson, R. L.; Balasas, T.; Callaghan, J.; Coombes, R. C.; Evans, J.; Hall, J. A.; Kinrade, S.; Jones, D.; Jones, P. S.; Jones, R.; Marshall, J. F.; Panico, M. B.; Shaw, J. A.; Steeg, P. S.; Sullivan, M.; Tong, W.; Westwell, A. D.; Ritchie, J. W. A.; Berg, R.; Drysdale, M.; Eccles, S.; Elvin, P.; Harris, A.; Ireson, C.; Machesky, L.; McLeod, R.; Muschel, R.; Newell, H.; Pittman, M.; Roman, B.; Santos, C.; Sibson, N.; Smith, A.; Waddell, I. Nat. Rev. Clin. Oncol. 2019, 16, 185.
doi: 10.1038/s41571-018-0134-8 pmid: 30514977 |
| [3] |
Ma, Q. X.; Xu, Q. Q.; Zhao, J. J.; Zhang, W. W.; Wang, Q.; Fang, J.; Lu, Z. M.; Liu, J.; Ma, L. N. Cancer Lett. 2021, 520, 243.
|
| [4] |
Bargh, J. D.; Isidro-Llobet, A.; Parker, J. S.; Spring, D. R. Chem. Soc. Rev. 2019, 48, 4361.
|
| [5] |
Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Signal Transduct. Target. Ther. 2022, 7, 93.
|
| [6] |
Paul, S.; Konig, M. F.; Pardoll, D. M.; Bettegowda, C.; Papadopoulos, N.; Wright, K. M.; Gabelli, S. B.; Ho, M.; van Elsas, A.; Zhou, S. Nat. Rev. Cancer 2024, 24, 399.
|
| [7] |
Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Acta Pharm. Sin. B 2023, 13, 498.
|
| [8] |
Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Int. J. Oncol. 2020, 57, 678.
doi: 10.3892/ijo.2020.5099 pmid: 32705178 |
| [9] |
Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D. G.; Zhang, W.-D. Nat. Prod. Rep. 2021, 38, 7.
|
| [10] |
Cooper, B. M.; Iegre, J.; O' Donovan, D. H.; Halvarsson, M. O.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 1480.
|
| [11] |
Dean, T. T.; Jelu-Reyes, J.; Allen, A. C.; Moore, T. W. J. Med. Chem. 2024, 67, 1641.
|
| [12] |
He, H.; Deng, X.; Wang, Z.; Chen, J. Eur. J. Med. Chem. 2025, 284, 117204.
|
| [13] |
Sagar, B.; Gupta, S.; Verma, S. K.; Reddy, Y. V. M.; Shukla, S. Eur. J. Med. Chem. 2025, 283, 117131.
|
| [14] |
Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; Liang, S.; Zeng, Y.; Liu, Z. J. Med. Chem. 2019, 62, 11108.
doi: 10.1021/acs.jmedchem.9b01126 pmid: 31735030 |
| [15] |
Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjornsson, B.; Rekdal, O.; Demaria, S.; Galluzzi, L. Trends Cancer 2021, 7, 557.
doi: 10.1016/j.trecan.2020.12.012 pmid: 33446447 |
| [16] |
Yin, H.; Chen, X.-T.; Chi, Q.-N.; Ma, Y.-N.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Acta Pharmacol. Sin. 2023, 44, 201.
|
| [17] |
Yin, H.; Fu, X.-Y.; Gao, H.-Y.; Ma, Y.-N.; Yao, J.-F.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Bioorg. Chem. 2023, 138, 106674.
|
| [18] |
Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. J. Med. Chem. 2024, 67, 3885.
|
| [19] |
Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; Qi, Y.-M.; Liu, L.; Gao, Y.-F. Angew. Chem. Int. Ed. 2015, 54, 11760.
|
| [20] |
Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; Zhao, W.; Wu, Y.; Qi, Y.; Liu, L.; Gao, Y. Angew. Chem. Int. Ed. 2020, 59, 15114.
|
| [21] |
Qi, Y. K.; Zheng, J. S.; Liu, L. Chem 2024, 10, 2390.
|
| [22] |
Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; Zhao, W.; Wu, Y.; Zhai, W.; Gao, Y. Acta Pharm. Sin. B 2024, 14, 1150.
|
| [23] |
Vrettos, E. I.; Mezo, G.; Tzakos, A. G. Beilstein J. Org. Chem. 2018, 14, 930.
|
| [24] |
Alas, M.; Saghaeidehkordi, A.; Kaur, K. J. Med. Chem. 2021, 64, 216.
|
| [25] |
Lindberg, J.; Nilvebrant, J.; Nygren, P.-A.; Lehmann, F. Molecules 2021, 26, 6042.
|
| [26] |
Li, Y.-J.; Fang, C.-B.; Wang, S.-S.; Chen, X.-Q.; Li, Y.; Liu, Q.; Qi, Y.-K.; Du, S.-S. Bioorgan. Med. Chem. 2024, 111, 117869.
|
| [27] |
Gong, L.; Zhao, H.; Liu, Y.; Wu, H.; Liu, C.; Chang, S.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Huang, W. Acta Pharm. Sin. B 2023, 13, 3659.
|
| [28] |
Hennrich, U.; Kopka, K. Pharmaceuticals 2019, 12, 114.
|
| [29] |
Strosberg, J. R.; Caplin, M. E.; Kunz, P. L.; Ruszniewski, P. B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E. M.; Yao, J. C.; Pavel, M. E.; Grande, E.; Van Cutsem, E.; Seregni, E.; Duarte, H.; Gericke, G.; Bartalotta, A.; Mariani, M. F.; Demange, A.; Mutevelic, S.; Krenning, E. P.; Investigators, N.-E.-. Lancet Oncol. 2021, 22, 1752.
doi: 10.1016/S1470-2045(21)00572-6 pmid: 34793718 |
| [30] |
Griffiths, G. L.; Vasquez, C.; Escorcia, F.; Clanton, J.; Lindenberg, L.; Mena, E.; Choyke, P. L. Adv. Drug Deliv. Rev. 2022, 181, 114086.
|
| [31] |
Huang, C. M.; Wu, Y. T.; Chen, S. T. Chem. Biol. 2000, 7, 453.
|
| [32] |
Gaviglio, L.; Gross, A.; Metzler-Nolte, N.; Ravera, M. Metallomics 2012, 4, 260.
doi: 10.1039/c2mt00171c pmid: 22310724 |
| [33] |
Lelle, M.; Kaloyanova, S.; Freidel, C.; Theodoropoulou, M.; Musheev, M.; Niehrs, C.; Stalla, G.; Peneva, K. Mol. Pharm. 2015, 12, 4290.
|
| [34] |
Ebaston, T. M.; Rozoysky, A.; Zaporozhets, A.; Bazyleyich, A.; Tuchinsky, H.; Marks, V.; Gellerman, G.; Patsenker, L. D. ChemMedChem 2019, 14, 1727.
doi: 10.1002/cmdc.201900464 pmid: 31403246 |
| [35] |
Rozovsky, A.; Ebaston, T. M.; Zaporozhets, A.; Bazylevich, A.; Tuchinsky, H.; Patsenker, L.; Gellerman, G. RSC Adv. 2019, 9, 32656.
doi: 10.1039/c9ra06334j |
| [36] |
Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Eur. J. Med. Chem. 2024, 265, 116119.
|
| [37] |
Zhou, J.; Li, Y.; Huang, W.; Shi, W.; Qian, H. Eur. J. Med. Chem. 2021, 224, 113712.
|
| [38] |
Deng, X.; Mai, R. Y.; Zhang, C. Y.; Yu, D. B.; Ren, Y. C.; Li, G.; Cheng, B. B.; Li, L.; Yu, Z. Q.; Chen, J. J. Eur. J. Med. Chem. 2021, 213, 113050.
|
| [39] |
Sharma, A.; Singh, L. R. Eur. J. Med. Chem. 2024, 271, 116456.
|
| [40] |
Qin, F.; Zhou, H.; Li, J.; Liu, J.; Wang, Y.; Bai, R.; Liu, S.; Manman, M.; Liu, T.; Gao, F.; Du, P.; Lu, X.; Chen, C. Eur. J. Pharmacol. 2021, 905, 174187.
|
| [41] |
Ding, J.; Wang, T.; Rong, Y.; He, C.; Chen, X. Chinese J. Chem. 2024, 42, 2957.
|
| [42] |
Huang, Y.; Luo, J.; Li, J.; Zhang, R.; Liu, X.; Fan, Q.; Huang, W. Acta Chim. Sinica 2024, 82, 903. (in Chinese)
|
|
(黄艳琴, 罗集文, 李佳启, 张瑞, 刘兴奋, 范曲立, 黄维, 化学学报, 2024, 82, 903.)
doi: 10.6023/A24040115 |
|
| [43] |
Li, M.; Zhang, J.; Liu, L.; Xu, S.; Liu, H. Acta Chim. Sinica 2024, 82, 856. (in Chinese)
|
|
(李梦丽, 张婕, 刘丽珍, 徐首红, 刘洪来, 化学学报, 2024, 82, 856.)
doi: 10.6023/A24040125 |
|
| [44] |
Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Nat. Rev. Drug Discov. 2017, 16, 315.
|
| [45] |
Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. J. Control. Release 2018, 275, 129.
|
| [46] |
White, B. H.; Whalen, K.; Kriksciukaite, K.; Alargova, R.; Yeung, T. A.; Bazinet, P.; Brockman, A.; DuPont, M.; Oller, H.; Lemelin, C.-A.; Soo, P. L.; Moreau, B.; Perino, S.; Quinn, J. M.; Sharma, G.; Shinde, R.; Sweryda-Krawiec, B.; Wooster, R.; Bilodeau, M. T. J. Med. Chem. 2019, 62, 2708.
|
| [47] |
Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Cells 2023, 12, 659.
|
| [48] |
Lee, J.; Choi, M. K.; Song, I. S. Pharmaceuticals 2023, 16, 802.
|
| [49] |
Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Mol. Asp. Med. 2023, 93, 101205.
|
| [50] |
Wang, S.; Sun, L.; Cao, H.; Zhong, Y.; Shao, Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese)
|
|
(王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.)
doi: 10.6023/A21050203 |
|
| [51] |
Guillier, F.; Orain, D.; Bradley, M. Chem. Rev. 2000, 100, 2091.
doi: 10.1021/cr980040+ pmid: 11749285 |
| [52] |
Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Chem. Rev. 2009, 109, 2455.
doi: 10.1021/cr800323s pmid: 19364121 |
| [53] |
Harris, P. W. R.; Kowalczyk, R.; Yang, S.-H.; Williams, G. M.; Brimble, M. A. J. Pept. Sci. 2014, 20, 186.
doi: 10.1002/psc.2595 pmid: 24353069 |
| [54] |
Skalska, J.; Andrade, V. M.; Cena, G. L.; Harvey, P. J.; Gaspar, D.; Mello, É.; Henriques, S. T.; Valle, J.; Gomes, V. M.; Conceiçao, K.; Castanho, M.; Andreu, D. J. Med. Chem. 2020, 63, 9391.
doi: 10.1021/acs.jmedchem.0c00543 pmid: 32787086 |
| [55] |
Wang, J. Y.; Dong, L. Y.; Liu, Y. N.; Chen, X. T.; Ma, Y. N.; Yin, H.; Du, S. S.; Qi, Y. K.; Wang, K. W. Chin. J. Org. Chem. 2021, 41, 2800. (in Chinese)
|
|
(王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.)
doi: 10.6023/cjoc202102045 |
|
| [56] |
Chen, X.-T.; Wang, J.-Y.; Ma, Y.-N.; Dong, L.-Y.; Jia, S.-X.; Yin, H.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K. J. Pept. Sci. 2022, 28, e3368.
|
| [57] |
Ma, Y.; Liu, Y. N.; Wang, J.; Chen, X.; Yin, H.; Chi, Q.; Jia, S.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2022, 42, 498. (in Chinese)
|
|
(马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 498.)
doi: 10.6023/cjoc202109003 |
|
| [58] |
Yin, H.; Chen, X.; Fu, X.; Ma, Y.; Xu, Y.; Zhang, T.; Liang, S.; Du, S.; Qi, Y.; Wang, K. Acta Chim. Sinica 2022, 80, 444. (in Chinese)
|
|
(尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威, 化学学报, 2022, 80, 444.)
doi: 10.6023/A21120580 |
|
| [59] |
Song, M.; Liu, Q.; Yao, J.-F.; Wang, Y.-T.; Ma, Y.-N.; Xu, H.; Yu, Q.-Y.; Li, Z.; Du, S.-S.; Qi, Y.-K. Bioorgan. Med. Chem. 2024, 107, 117760.
|
| [60] |
Spears, R. J.; McMahon, C.; Chudasama, V. Chem. Soc. Rev. 2021, 50, 11098.
|
| [61] |
Pan, M.; Gao, S.; Zheng, Y.; Tan, X.; Lan, H.; Tan, X.; Sun, D.; Lu, L.; Wang, T.; Zheng, Q.; Huang, Y.; Wang, J.; Liu, L. J. Am. Chem. Soc. 2016, 138, 7429.
|
| [62] |
Zuo, C.; Shi, W.-W.; Chen, X.-X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang, G.-M.; Bierer, D.; Liu, L. Sci. China: Chem. 2019, 62, 1371.
|
| [63] |
Liang, L.-J.; Chu, G.-C.; Qu, Q.; Zuo, C.; Mao, J.; Zheng, Q.; Chen, J.; Meng, X.; Jing, Y.; Deng, H.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2021, 60, 17171.
|
| [64] |
Ai, H.; Chu, G.-C.; Gong, Q.; Tong, Z.-B.; Deng, Z.; Liu, X.; Yang, F.; Xu, Z.; Li, J.-B.; Tian, C.; Liu, L. J. Am. Chem. Soc. 2022, 144, 18329.
|
| [65] |
Ai, H.; Sun, M.; Liu, A.; Sun, Z.; Liu, T.; Cao, L.; Liang, L.; Qu, Q.; Li, Z.; Deng, Z.; Tong, Z.; Chu, G.; Tian, X.; Deng, H.; Zhao, S.; Li, J.-B.; Lou, Z.; Liu, L. Nat. Chem. Biol. 2022, 18, 972.
|
| [66] |
Zhang, B.; Zheng, Y.; Chu, G.; Deng, X.; Wang, T.; Shi, W.; Zhou, Y.; Tang, S.; Zheng, J.-S.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202306270.
|
| [67] |
Zhao, R.; Shi, P.; Cui, J.-b.; Shi, C.; Wei, X.-X.; Luo, J.; Xia, Z.; Shi, W.-W.; Zhou, Y.; Tang, J.; Tian, C.; Meininghaus, M.; Bierer, D.; Shi, J.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202216365.
|
| [68] |
You, Y.; Xu, Z.; Chen, Y. Drug Deliv. 2018, 25, 448.
|
| [69] |
Randelovic, I.; Schuster, S.; Kapuvari, B.; Fossati, G.; Steinkuehler, C.; Mezo, G.; Tovari, J. Int. J. Mol. Sci. 2019, 20, 4763.
|
| [70] |
He, Y.; Yuan, X. M.; Lei, P.; Wu, S.; Xing, W.; Lan, X. L.; Zhu, H. F.; Huang, T.; Wang, G. B.; An, R.; Zhang, Y. X.; Shen, G. X. Acta Pharmacol. Sin. 2009, 30, 1053.
|
| [71] |
Zhang, J.; Jin, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Mol. Pharm. 2010, 7, 1159.
|
| [72] |
Sanwick, A. M.; Haugh, K. N.; Williams, E. J.; Perry, K. A.; Thiele, N. A.; Chaple, I. F. EJNMMI Radiopharm. Chem. 2024, 9, 88.
|
| [73] |
Zhou, Y.; Liu, X.; Xue, J.; Liu, L.; Liang, T.; Li, W.; Yang, X.; Hou, X.; Fang, H. J. Med. Chem. 2020, 63, 4701.
doi: 10.1021/acs.jmedchem.9b02161 pmid: 32267687 |
| [74] |
Lu, M.; Xing, H.; Shao, W.; Wu, P.; Fan, Y.; He, H.; Barth, S.; Zheng, A.; Liang, X.-J.; Huang, Y. Acta Pharm. Sin. B 2023, 13, 3945.
|
| [75] |
Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem. Int. Ed. 2011, 50, 7645.
|
| [76] |
Fang, G.-M.; Wang, J.-X.; Liu, L. Angew. Chem. Int. Ed. 2012, 51, 10347.
|
| [77] |
Zheng, J.-S.; Tang, S.; Qi, Y.-K.; Wang, Z.-P.; Liu, L. Nat. Protoc. 2013, 8, 2483.
|
| [78] |
Dong, S.; Zheng, J.-S.; Li, Y.; Wang, H.; Chen, G.; Chen, Y.; Fang, G.; Guo, J.; He, C.; Hu, H.; Li, X.; Li, Y.; Li, Z.; Pan, M.; Tang, S.; Tian, C.; Wang, P.; Wu, B.; Wu, C.; Zhao, J.; Liu, L. Sci. China: Chem. 2024, 67, 1060.
|
| [79] |
Chi, Q.-N.; Jia, S.-X.; Yin, H.; Wang, L.-E.; Fu, X.-Y.; Ma, Y.-N.; Sun, M.-P.; Qi, Y.-K.; Li, Z.; Du, S.-S. Bioorg. Chem. 2023, 134, 106451.
|
| [80] |
Kobayashi, K.; Taguchi, A.; Cui, Y.; Shida, H.; Muguruma, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y. Eur. J. Org. Chem. 2021, 2021, 956.
|
| [81] |
Yin, H.; Fu, X.; Gao, H.; Gao, H.; Ma, Y.; Chen, X.; Zhang, X.; Du, S.-S.; Qi, Y.-K. Front. Oncol. 2023, 12, 1028600.
|
| [82] |
Qiao, Z.; Qi, H.; Zhang, H.; Zhou, Q.; Wei, N.; Zhang, Y.; Wang, K. Anal. Chem. 2020, 92, 1934.
|
| [1] | Jian Zheng, Jin-Hong Lin, Ji-Chang Xiao. Difluorocarbene-based Trifluoromethylthiolation of Aryl and Alkenyl Iodides [J]. Acta Chimica Sinica, 2024, 82(2): 115-118. |
| [2] | Hao Yin, Xitong Chen, Xingyan Fu, Yannan Ma, Yimei Xu, Te Zhang, Shuai Liang, Shanshan Du, Yunkun Qi, Kewei Wang. Efficient Chemical Synthesis and Oxidative Folding Studies of Scorpion Toxin Peptide WaTx [J]. Acta Chimica Sinica, 2022, 80(4): 444-452. |
| [3] | Hao-Nan Qin, Zhao-Yang Wang, Shuang-Quan Zang. Photoluminescence and Electrochemical Sensing of Atomically Precise Cu13 Cluster [J]. Acta Chimica Sinica, 2021, 79(8): 1037-1041. |
| [4] | Shen Yanglin, Jin Junling, Duan Guangxiong, Xie Yunpeng, Lu Xing. Formation of Spindle-Like Ag58 Cluster Induced by Isomerization of [Ag14] [J]. Acta Chimica Sinica, 2020, 78(11): 1255-1259. |
| [5] | Yang, Junhang, Fu, Xiaobo, Lu, Zenghui, Zhu, Gangguo. Visible-Light Photocatalytic Remote Thiolation of Aldehydes Triggered by Sulfonylation of Alkenes With Thiosulfonates [J]. Acta Chimica Sinica, 2019, 77(9): 901-905. |
| [6] | Chen Dong, Ji Meishan, Yao Yingming, Zhu Chen. Difunctionalization of Unactivated Alkenes through SCF3 Radical-triggered Distal Functional Group Migration [J]. Acta Chimica Sinica, 2018, 76(12): 951-955. |
| [7] | Li Shu-Sen, Wang Jianbo. Recent Advance in Asymmetric Trifluoromethylthiolation [J]. Acta Chim. Sinica, 2018, 76(12): 913-924. |
| [8] | Gao Xiao-Fei, He Peng, Chen Huanwen. Study on the Interaction Between Water Radical Cations and Bis(2-hydroxyethyl) Disulfide at Ambient Temperature and Pressure Using Mass Spectrometry [J]. Acta Chim. Sinica, 2018, 76(10): 802-806. |
| [9] | Zhang Panpan, Lu Long, Shen Qilong. Recent Progress on Direct Trifluoromethylthiolating Reagents and Methods [J]. Acta Chim. Sinica, 2017, 75(8): 744-769. |
| [10] | Zhang Yiwe, Ma Xuelu, Zhang Xin, Lei Ming. Theoretical Study on N-N Activation by Thiolate-bridged Dinuclear Dinitrogen Transition-metal Complexes [J]. Acta Chim. Sinica, 2016, 74(4): 340-350. |
| [11] | Xu Jiabin, Chen Pinhong, Ye Jinxing, Liu Guosheng. Palladium-Catalyzed Trifluoromethylthiolation of Arenes via C-H Activation [J]. Acta Chimica Sinica, 2015, 73(12): 1294-1297. |
| [12] | Cui Xiaoteng, Lv Yuyang, Liu Ying, Wu Boyue. Aqueous Synthesis of Near-Infrared CdTe Quantum Dots for Biothiols Detection in Biological Fluids [J]. Acta Chimica Sinica, 2014, 72(1): 75-82. |
| [13] | Ran Runxin, Fan Xiaoli, Liu Yan, Yang Yongliang. Density Functional Theory Studies on the Adsorption of Methanethiol Molecule on Au(111) Surface at Different Coverage [J]. Acta Chimica Sinica, 2013, 71(05): 829-836. |
| [14] | Han Cong, Xu Zhe, Diao Chunhua, Chen Xin, Liu Jing, Guo Minjie, Fan Zhi. Self-assembly Behavior of Interlocked Helical Supramolecule of Cyclodextrin Modified by 2-Furanmethanethiol [J]. Acta Chimica Sinica, 2013, 71(03): 439-442. |
| [15] | MIN Jia-Xiang, FAN Xiao-Li, CHENG Qian-Zhong, CHI Qiong. Density Functional Theory Studies on the Adsorption of Methane-thiol Molecule on Au(111) Surface [J]. Acta Chimica Sinica, 2011, 69(07): 789-797. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||