Acta Chimica Sinica ›› 2026, Vol. 84 ›› Issue (1): 20-29.DOI: 10.6023/A25050188 Previous Articles Next Articles
Article
苏健利, 李长旭, 陈春明, 安会丽, 于峰*( ), 陈树伟, 潘大海*(
), 陈树伟, 潘大海*( ), 闫晓亮, 李瑞丰
), 闫晓亮, 李瑞丰
投稿日期:2025-05-26
发布日期:2025-08-11
基金资助:
Jianli Su, Changxu Li, Chunming Chen, Huili An, Feng Yu*( ), Shuwei Chen, Dahai Pan*(
), Shuwei Chen, Dahai Pan*( ), Xiaoliang Yan, Ruifeng Li
), Xiaoliang Yan, Ruifeng Li
Received:2025-05-26
Published:2025-08-11
Contact:
* E-mail: yufeng@tyut.edu.cn (F. Yu);pandahai@foxmail.com (DH. Pan)
Supported by:Share
Jianli Su, Changxu Li, Chunming Chen, Huili An, Feng Yu, Shuwei Chen, Dahai Pan, Xiaoliang Yan, Ruifeng Li. Support Performance Optimization and Pt High-Dispersion Mechanism for Methylcyclohexane (MCH) High-Efficiency Dehydrogenation Catalyst Pt/Al2O3[J]. Acta Chimica Sinica, 2026, 84(1): 20-29.
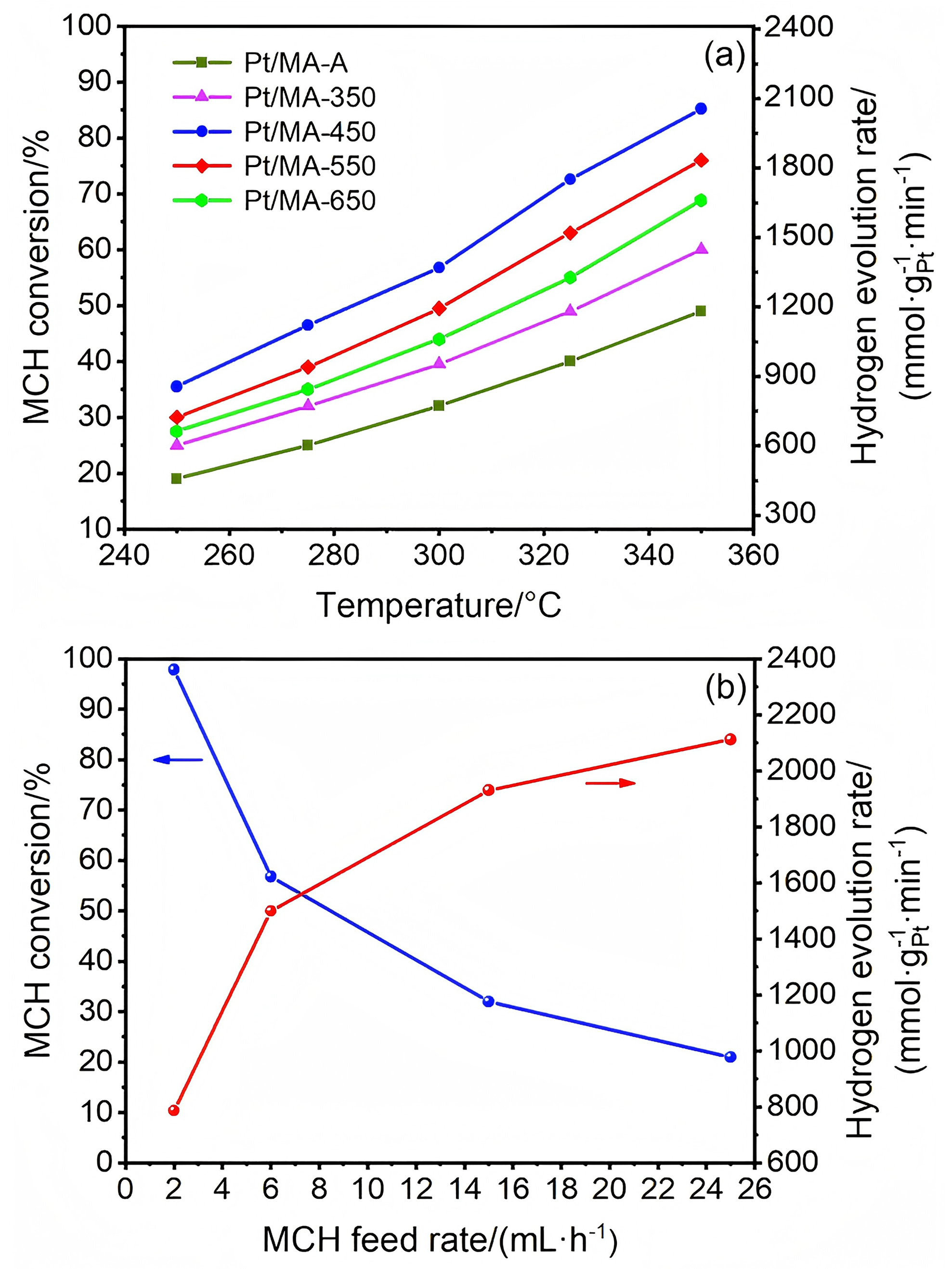
| Catalyst | w(Pt)/% | Temperature/℃ | WHSV/h–1 | H2 evolution rate/(mmol•gPt–1•min–1) | Ref. |
|---|---|---|---|---|---|
| Pt/Mg-Al-O | 0.5 | 300/350 | 9.48 | 461.5/1892 | [ |
| Pt/Al2O3 | 1.5 | 300 | 28.4 | 656 | [ |
| B(Pt-n)/Al2O3 | 1 | 300 | 23.7 | 984 | [ |
| 1%Pt/Clu-350 | 1 | 320 | 47.4 | 1118.8 | [ |
| Pt1Sn6@S-1 | 0.4 | 350 | 75.84 | 1343 | [ |
| Pt/SG-9 | 0.5 | 300 | 98.75 | 2081 | [ |
| Pt/MA-450 | 0.5 | 300 | 98.75 | 2112 | this work |
| Catalyst | w(Pt)/% | Temperature/℃ | WHSV/h–1 | H2 evolution rate/(mmol•gPt–1•min–1) | Ref. |
|---|---|---|---|---|---|
| Pt/Mg-Al-O | 0.5 | 300/350 | 9.48 | 461.5/1892 | [ |
| Pt/Al2O3 | 1.5 | 300 | 28.4 | 656 | [ |
| B(Pt-n)/Al2O3 | 1 | 300 | 23.7 | 984 | [ |
| 1%Pt/Clu-350 | 1 | 320 | 47.4 | 1118.8 | [ |
| Pt1Sn6@S-1 | 0.4 | 350 | 75.84 | 1343 | [ |
| Pt/SG-9 | 0.5 | 300 | 98.75 | 2081 | [ |
| Pt/MA-450 | 0.5 | 300 | 98.75 | 2112 | this work |
| Catalyst | Dispersion/% | Particle size/nm | Metal surface area/ (m2•g–1) |
|---|---|---|---|
| Pt/MA-A | 46.7 | 2.05 | 116.8 |
| Pt/MA-350 | 49.9 | 1.89 | 123.1 |
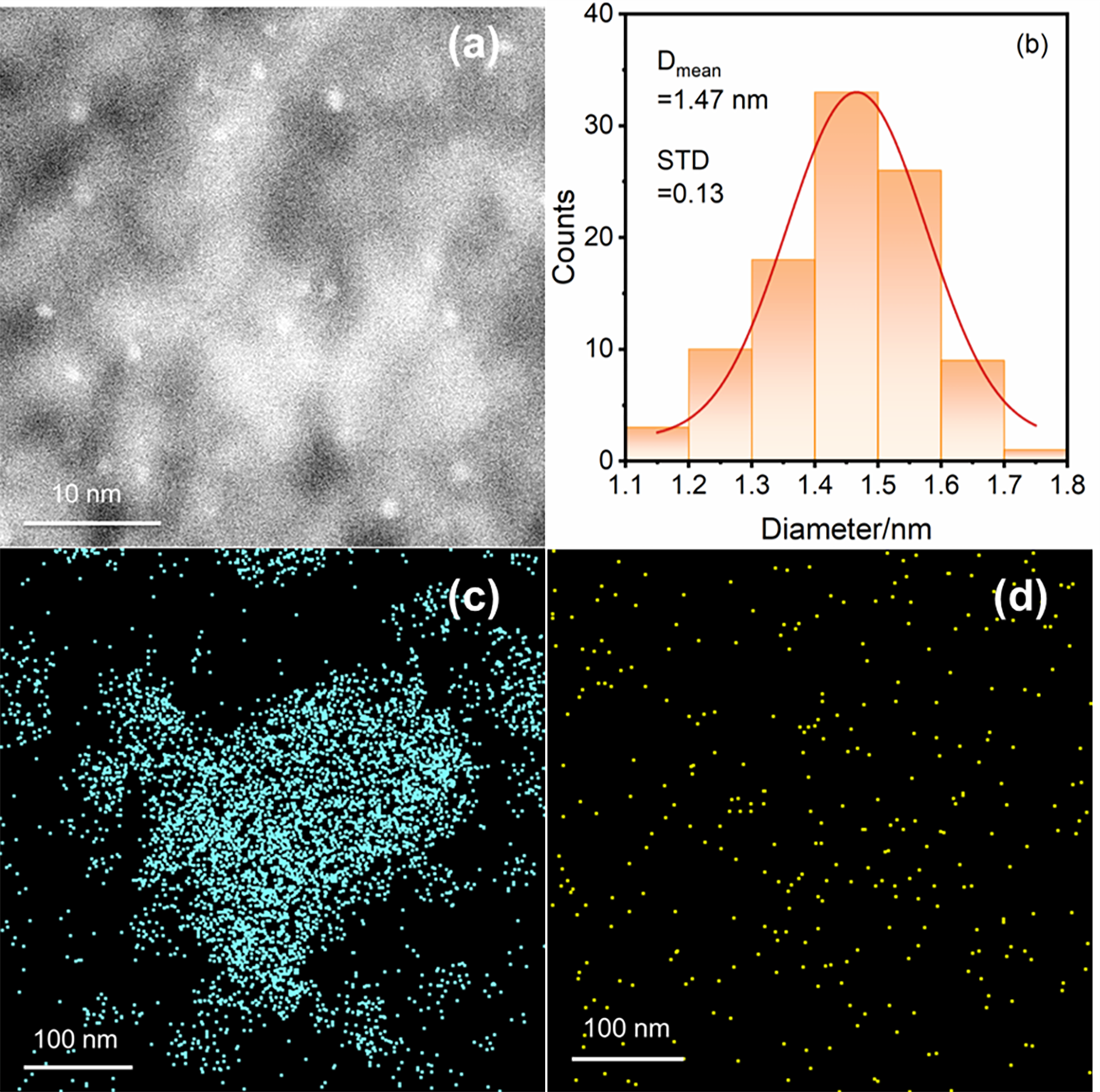
| Pt/MA-450 | 63.6 | 1.49 | 157.1 |
| Pt/MA-550 | 56.8 | 1.66 | 140.1 |
| Pt/MA-650 | 53.6 | 1.76 | 132.3 |
| Catalyst | Dispersion/% | Particle size/nm | Metal surface area/ (m2•g–1) |
|---|---|---|---|
| Pt/MA-A | 46.7 | 2.05 | 116.8 |
| Pt/MA-350 | 49.9 | 1.89 | 123.1 |
| Pt/MA-450 | 63.6 | 1.49 | 157.1 |
| Pt/MA-550 | 56.8 | 1.66 | 140.1 |
| Pt/MA-650 | 53.6 | 1.76 | 132.3 |
| Support | SBET/(m2•g–1) | Vp/(cm3•g–1) | Dp/nm |
|---|---|---|---|
| MA-A | 367 | 0.55 | 4.34 |
| MA-350 | 401 | 0.75 | 6.93 |
| MA-450 | 550 | 1.21 | 7.02 |
| MA-550 | 453 | 0.95 | 6.78 |
| MA-650 | 349 | 0.61 | 5.81 |
| Support | SBET/(m2•g–1) | Vp/(cm3•g–1) | Dp/nm |
|---|---|---|---|
| MA-A | 367 | 0.55 | 4.34 |
| MA-350 | 401 | 0.75 | 6.93 |
| MA-450 | 550 | 1.21 | 7.02 |
| MA-550 | 453 | 0.95 | 6.78 |
| MA-650 | 349 | 0.61 | 5.81 |
| Support | x(O)/% | x(Al)/% | x(OL)/% | x(OV)/% | x(OH)/% |
|---|---|---|---|---|---|
| MA-450 | 55.39 | 29.81 | 21.16 | 24.32 | 9.91 |
| MA-550 | 54.76 | 30.37 | 21.29 | 24.43 | 9.04 |
| MA-650 | 54.93 | 30.83 | 22.41 | 24.88 | 7.64 |
| Support | x(O)/% | x(Al)/% | x(OL)/% | x(OV)/% | x(OH)/% |
|---|---|---|---|---|---|
| MA-450 | 55.39 | 29.81 | 21.16 | 24.32 | 9.91 |
| MA-550 | 54.76 | 30.37 | 21.29 | 24.43 | 9.04 |
| MA-650 | 54.93 | 30.83 | 22.41 | 24.88 | 7.64 |
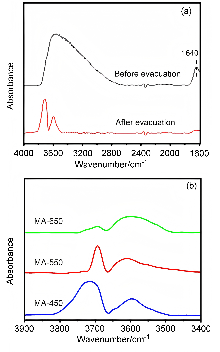
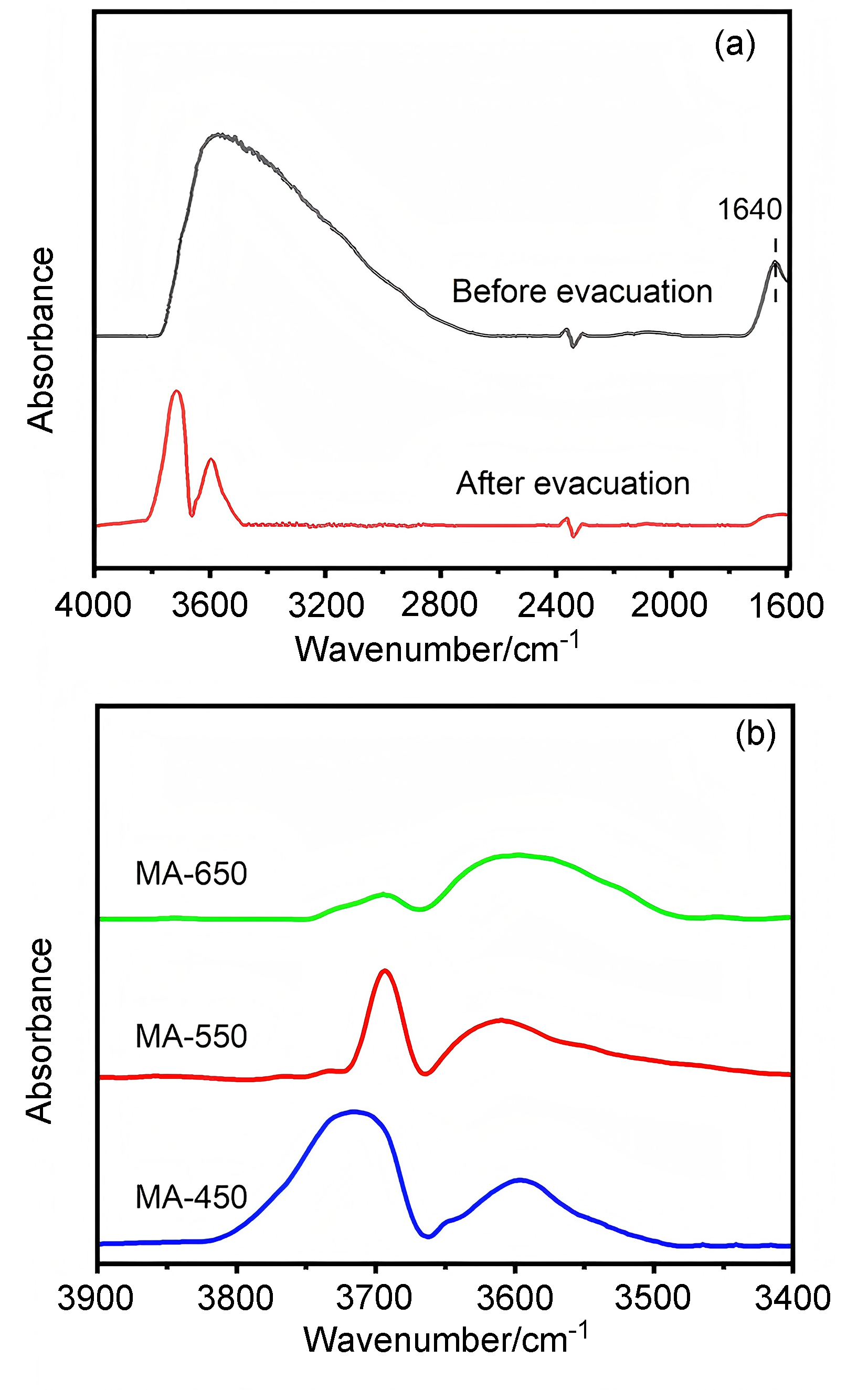
| [1] |
doi: 10.1016/j.cej.2023.141918 |
| [2] |
doi: 10.1016/j.renene.2024.120911 |
| [3] |
doi: 10.1016/j.ijhydene.2025.02.257 |
| [4] |
doi: 10.1016/j.ijhydene.2023.04.024 |
| [5] |
doi: 10.1016/j.ijhydene.2025.04.371 |
| [6] |
doi: 10.1016/j.ijhydene.2024.12.104 |
| [7] |
doi: 10.6023/A24030077 |
|
(李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚, 化学学报, 2024, 82, 748.)
doi: 10.6023/A24030077 |
|
| [8] |
doi: 10.1016/j.ijhydene.2024.08.331 |
| [9] |
doi: 10.1016/j.enconman.2023.117049 |
| [10] |
doi: 10.1016/j.cattod.2024.114688 |
| [11] |
doi: 10.1016/j.fuel.2023.130607 |
| [12] |
doi: 10.1039/D3CY01568H |
| [13] |
doi: 10.1016/j.apsusc.2023.157134 |
| [14] |
doi: 10.6023/A23120546 |
|
(张强, 王欢, 王帅, 王园园, 张梅, 宋华, 化学学报, 2024, 82, 287.)
doi: 10.6023/A23120546 |
|
| [15] |
doi: 10.1016/j.ijhydene.2024.06.101 |
| [16] |
doi: 10.1016/j.ijhydene.2024.12.466 |
| [17] |
doi: 10.1038/s41467-024-55370-z |
| [18] |
doi: 10.1016/j.ijhydene.2021.05.056 |
| [19] |
doi: 10.1016/j.ijhydene.2022.08.085 |
| [20] |
doi: 10.1016/j.ijhydene.2025.03.459 |
| [21] |
doi: 10.1021/acssuschemeng.4c09762 |
| [22] |
doi: 10.1038/s41467-022-28607-y |
| [23] |
doi: 10.1016/j.fuel.2024.132851 |
| [24] |
doi: 10.6023/A25010018 |
|
(陈建华, 姜兰, 曾杨, 谢颂海, 裴燕, 乔明华, 化学学报, 2025, 83, 332.)
doi: 10.6023/A25010018 |
|
| [25] |
doi: 10.1016/j.cej.2024.152100 |
| [26] |
|
|
(丁心湄, 梁艳丽, 张海龙, 赵明, 王健礼, 陈耀强, 物理化学学报, 2022, 38, 76.)
|
|
| [27] |
doi: 10.6023/A24070217 |
|
(王帅, 宋华, 化学学报, 2024, 82, 979.)
doi: 10.6023/A24070217 |
|
| [28] |
|
| [29] |
doi: 10.1021/acs.jpcc.7b02498 |
| [30] |
doi: 10.1021/la990604k |
| [31] |
doi: 10.1021/acscatal.0c03091 |
| [32] |
doi: 10.1039/C8TA08262F |
| [33] |
doi: 10.1016/j.apcatb.2025.125321 |
| [34] |
doi: 10.1016/j.jcat.2006.08.024 |
| [35] |
doi: 10.1016/j.apcatb.2024.124342 |
| [36] |
doi: 10.1021/acs.est.2c01278 |
| [37] |
doi: 10.1039/D1CY01149A |
| [38] |
doi: 10.1016/j.jece.2025.115407 |
| [39] |
doi: 10.1016/j.cattod.2013.08.003 |
| [1] | Xiaoxuan Duan, Hengyan Wang, Dahai Pan, Shuwei Chen, Feng Yu, Xiaoliang Yan, Ruifeng Li. Designed Synthesis of SBA-15 Supported Sulfated Zirconia Solid Acid Materials and Their Catalytic Performance for Transesterification Reaction [J]. Acta Chimica Sinica, 2024, 82(7): 755-762. |
| [2] | Quanzheng Deng, Wenting Mao, Lu Han. Structural Solution of Porous Materials on the Mesostructural Scale by Electron Microscopy [J]. Acta Chimica Sinica, 2022, 80(8): 1203-1216. |
| [3] | Shao Yue, Ma Yong. Amino Group Surface-functionalized Ordered Mesoporous Materials:One-pot Synthesis, Heavy-metal Ion and CO2 Adsorption [J]. Acta Chimica Sinica, 2012, 70(18): 1957-1962. |
| [4] | WANG Ye-Hong, TAN Juan, LIU Jing, CHEN Ying, LI Xu-Ying. Removal of Template from Mesostructured Nickel Phosphates by Solvent Extraction [J]. Acta Chimica Sinica, 2010, 68(23): 2471-2476. |
| [5] | ZHU Jin-Yu, SHEN Yi, WU Long, GAN Si-Wen, CHEN An-Qi, SHEN Zhu-Ping, PAN Xiao-Qing, CHEN Yong. Studies on the Synthesis of Organic/Inorganic Hybrid Mesoporous Silica Membrane and Its Adsorption Behavior of Enzyme [J]. Acta Chimica Sinica, 2010, 68(21): 2231-2237. |
| [6] | SONG Lin, SHU Da-Zhang, SUN Xiao-Yu, HONG Shi-Long, SUN Dong-Mei. Multi-level assembly and synthesis of Spherical meso-level outlet hydroapatite nanoparticles [J]. Acta Chimica Sinica, 2009, 67(23): 2697-2702. |
| [7] | . A Novel Method for Synthesis of Zr-Ce-SBA-15 Mesoporous Materials with Hexagonal-platelet Morphology [J]. Acta Chimica Sinica, 2009, 67(11): 1271-1275. |
| [8] | TIAN Bo-Shi, YANG Chun*. Preparation and Characterization of Thermo-sensitive Mesoporous PNIPAAm/SBA-15 Composite [J]. Acta Chimica Sinica, 2008, 66(5): 505-510. |
| [9] | XU, Wu-Jun a,b GAO, Qiang a,b XU, Yao *,a WU, Dong a SUN, Yu-Han a. SBA-15 Tablet Coated with HPMCP for pH-Sensitive Drug Release System [J]. Acta Chimica Sinica, 2008, 66(14): 1658-1662. |
| [10] | CAO Jie-Ming*,1; WU Wei; CHEN Yu2; LIU Jing-Song; CAO Yu-Lin; HE Jian-Ping; TANG Ya-Wen2; YANG Chun2; LU Tian-Hong2. Preparation and Properties of New Anode Material Pt-Ru/CMK-3 [J]. Acta Chimica Sinica, 2007, 65(12): 1117-1122. |
| [11] | ZHANG Cun-Man*,1, LIU Qian2, XU Zheng. Preparation and Performance Study of Ordered Silicon Oxynitride MCM-41 Molecular Sieves with Basic Catalytic Activity [J]. Acta Chimica Sinica, 2006, 64(4): 313-319. |
| [12] | KONG Ling-Dong, LIU Su2, YAN Xue-Wu, HE He-Yong, LI Quan-Zhi*1. Synthesis of Highly Thermally and Hydrothermally Stable MCM-48 in Buffer Solution [J]. Acta Chimica Sinica, 2005, 63(13): 1241-1244. |
| [13] | ZHAI Shang-Ru, WEI Wei, WU Dong, SUN Yu-Han. Two-step Route to Mesoporous Al-MSU-X [J]. Acta Chimica Sinica, 2004, 62(4): 442-444. |
| [14] | SHI Qi-Hui, YANG Hai-Feng, CHENG Yan, YAN Yan, CHEN Ying, TU Bo, ZHAO Dong-Yuan. Electrodeposition of Novel Nanostructured Materials Using Mesoporous Silica Thin Films as Templates [J]. Acta Chimica Sinica, 2004, 62(20): 2021-2024. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||