Acta Chimica Sinica ›› 2026, Vol. 84 ›› Issue (1): 30-42.DOI: 10.6023/A25060224 Previous Articles Next Articles
Article
姚志豪a,b, 张巍a,b,*( ), 周昭仪a,b, 李丹聪a,b, 张凯凯a,b, 刘涛a,b, 胡文凯a,b, 程守安a,b, 胡铭轩a,b, 刘昱佳a,b
), 周昭仪a,b, 李丹聪a,b, 张凯凯a,b, 刘涛a,b, 胡文凯a,b, 程守安a,b, 胡铭轩a,b, 刘昱佳a,b
投稿日期:2025-06-17
发布日期:2025-08-18
基金资助:
Zhihao Yaoa,b, Wei Zhanga,b,*( ), Zhaoyi Zhoua,b, Dancong Lia,b, Kaikai Zhanga,b, Tao Liua,b, Wenkai Hua,b, Shouan Chenga,b, Mingxuan Hua,b, Yujia Liua,b
), Zhaoyi Zhoua,b, Dancong Lia,b, Kaikai Zhanga,b, Tao Liua,b, Wenkai Hua,b, Shouan Chenga,b, Mingxuan Hua,b, Yujia Liua,b
Received:2025-06-17
Published:2025-08-18
Contact:
* E-mail: weizhang@csust.edu.cn
Supported by:Share
Zhihao Yao, Wei Zhang, Zhaoyi Zhou, Dancong Li, Kaikai Zhang, Tao Liu, Wenkai Hu, Shouan Cheng, Mingxuan Hu, Yujia Liu. Study on Synergistic Modulation of LaMnO3 Electronic Structure and CO Selective Catalytic Reduction Reaction Mechanism via Sr/Fe[J]. Acta Chimica Sinica, 2026, 84(1): 30-42.
| dC-O/nm | dC-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-CO-Mn01 | 0.114 | 0.211 | –0.35 |
| LaSrMnO3-CO-Mn01 | 0.114 | 0.207 | –0.44 |
| LaMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn3 | 0.114 | 0.212 | –0.30 |
| LaMnFeO3-CO-Fe | 0.116 | 0.175 | –0.85 |
| LaSrMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.49 |
| LaSrMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.41 |
| LaSrMnFeO3-CO-Mn3 | 0.114 | 0.208 | –0.31 |
| LaSrMnFeO3-CO-Fe | 0.116 | 0.174 | –0.63 |
| dC-O/nm | dC-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-CO-Mn01 | 0.114 | 0.211 | –0.35 |
| LaSrMnO3-CO-Mn01 | 0.114 | 0.207 | –0.44 |
| LaMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn3 | 0.114 | 0.212 | –0.30 |
| LaMnFeO3-CO-Fe | 0.116 | 0.175 | –0.85 |
| LaSrMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.49 |
| LaSrMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.41 |
| LaSrMnFeO3-CO-Mn3 | 0.114 | 0.208 | –0.31 |
| LaSrMnFeO3-CO-Fe | 0.116 | 0.174 | –0.63 |
| Q(Sr)/e | Q(Fe)/e | Q(C)/e | Q(O)/e | Q(Mn/Fe)/e | ΔQCO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-CO | — | — | –0.292 | 0.246 | –1.659 | –0.046 |
| LaSrMnO3-CO | –1.659 | — | –0.061 | 0.022 | –1.710 | –0.039 |
| LaMnFeO3-CO | — | –1.364 | –0.120 | 0.238 | –1.544 | 0.118 |
| LaSrMnFeO3-CO | –1.660 | –1.436 | –0.126 | 0.236 | –1.608 | 0.110 |
| Q(Sr)/e | Q(Fe)/e | Q(C)/e | Q(O)/e | Q(Mn/Fe)/e | ΔQCO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-CO | — | — | –0.292 | 0.246 | –1.659 | –0.046 |
| LaSrMnO3-CO | –1.659 | — | –0.061 | 0.022 | –1.710 | –0.039 |
| LaMnFeO3-CO | — | –1.364 | –0.120 | 0.238 | –1.544 | 0.118 |
| LaSrMnFeO3-CO | –1.660 | –1.436 | –0.126 | 0.236 | –1.608 | 0.110 |
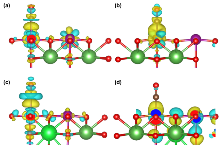
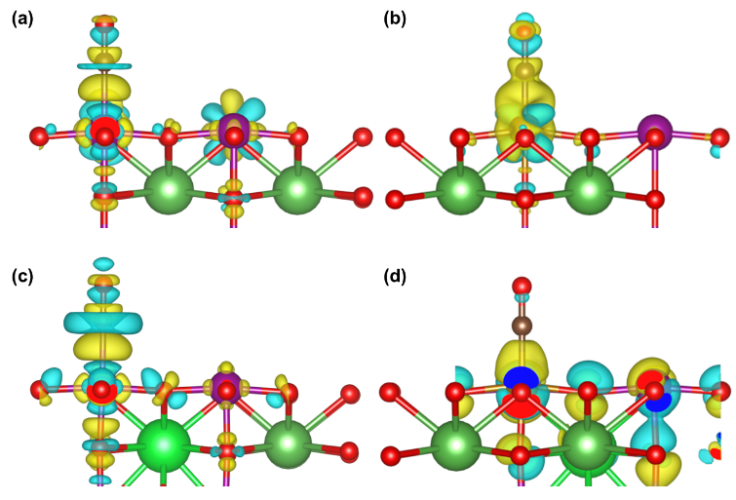
| dN-O/nm | dN-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-NO-Mn01 | 0.118 | 0.165 | –1.88 |
| LaSrMnO3-NO-Mn01 | 0.117 | 0.163 | –1.76 |
| LaMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.93 |
| LaMnFeO3-NO-Mn2 | 0.117 | 0.165 | –1.93 |
| LaMnFeO3-NO-Mn3 | 0.117 | 0.164 | –1.83 |
| LaMnFeO3-NO-Fe | 0.117 | 0.172 | –1.85 |
| LaSrMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.79 |
| LaSrMnFeO3-NO-Mn2 | 0.117 | 0.164 | –1.78 |
| LaSrMnFeO3-NO-Mn3 | 0.117 | 0.163 | –1.74 |
| LaSrMnFeO3-NO-Fe | 0.116 | 0.163 | –1.54 |
| dN-O/nm | dN-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-NO-Mn01 | 0.118 | 0.165 | –1.88 |
| LaSrMnO3-NO-Mn01 | 0.117 | 0.163 | –1.76 |
| LaMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.93 |
| LaMnFeO3-NO-Mn2 | 0.117 | 0.165 | –1.93 |
| LaMnFeO3-NO-Mn3 | 0.117 | 0.164 | –1.83 |
| LaMnFeO3-NO-Fe | 0.117 | 0.172 | –1.85 |
| LaSrMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.79 |
| LaSrMnFeO3-NO-Mn2 | 0.117 | 0.164 | –1.78 |
| LaSrMnFeO3-NO-Mn3 | 0.117 | 0.163 | –1.74 |
| LaSrMnFeO3-NO-Fe | 0.116 | 0.163 | –1.54 |
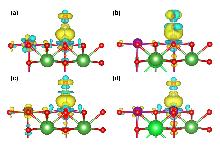
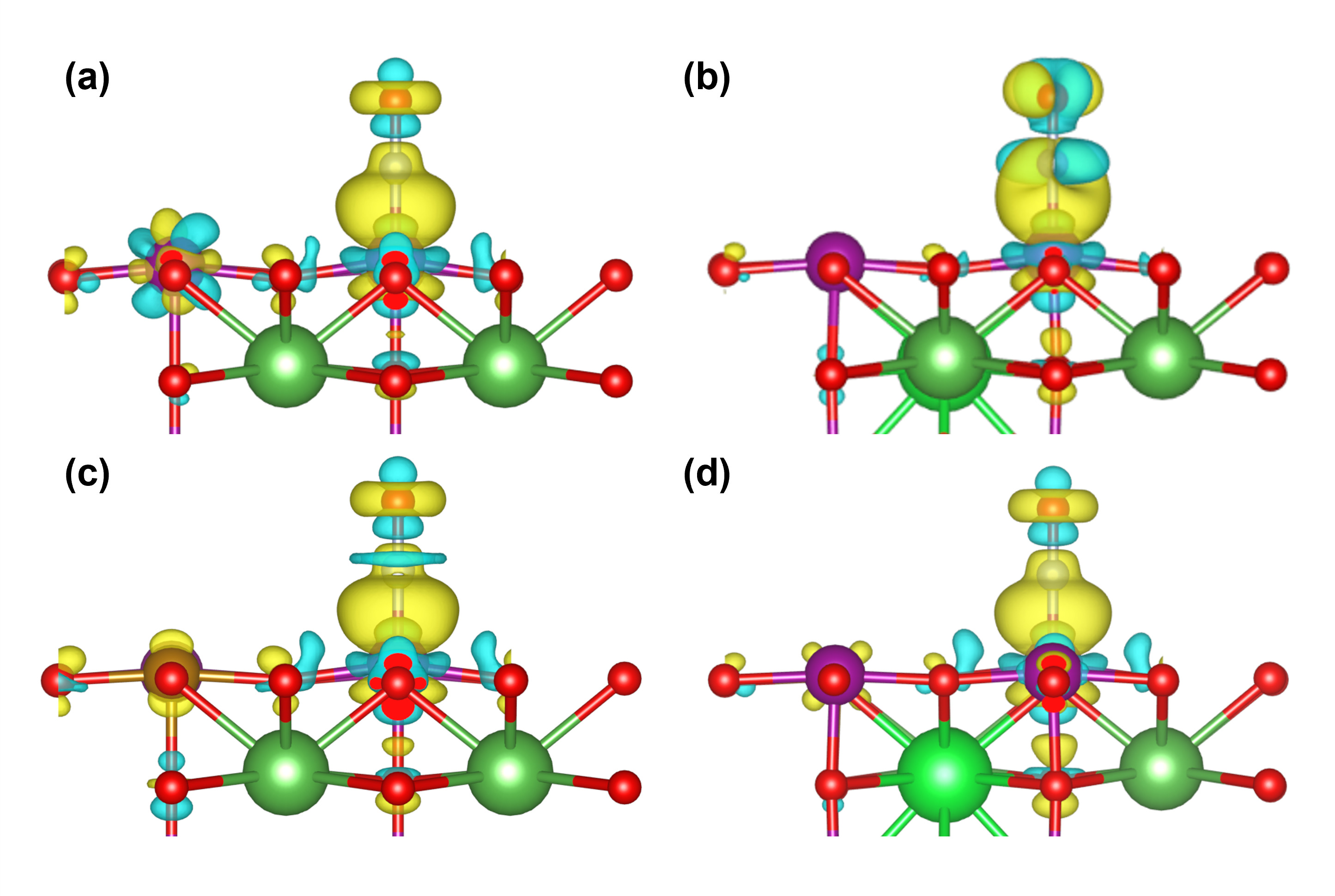
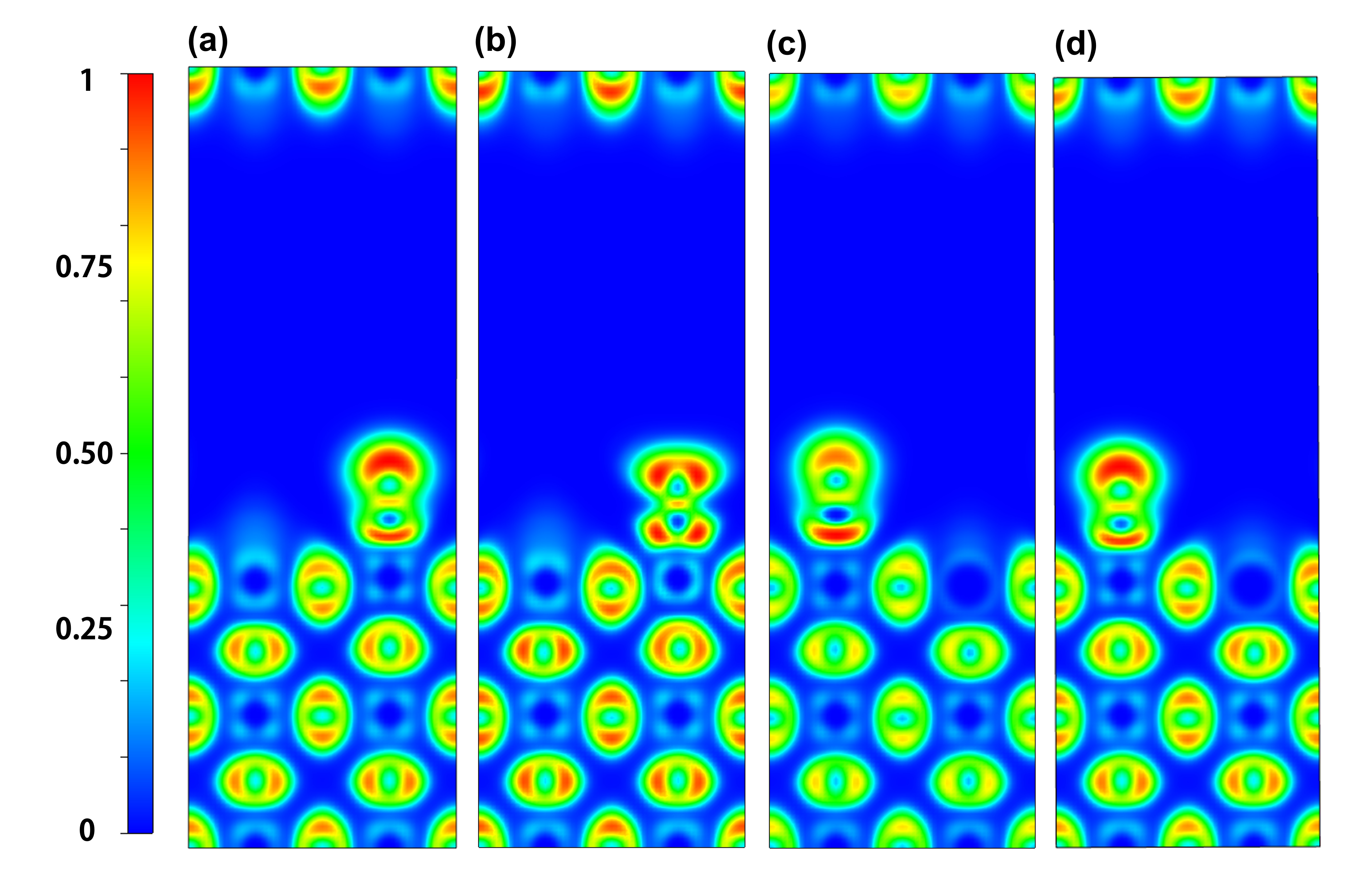
| Q(Sr)/e | Q(Fe)/e | Q(N)/e | Q(O)/e | Q(Mn)/e | ΔQNO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-NO | — | — | 0.112 | 0.135 | –1.636 | 0.245 |
| LaSrMnO3-NO | –1.664 | — | 0.106 | 0.126 | –1.634 | 0.226 |
| LaMnFeO3-NO | — | –1.601 | 0.110 | 0.146 | –1.641 | 0.256 |
| LaSrMnFeO3-NO | –1.658 | –1.591 | 0.148 | 0.101 | –1.698 | 0.249 |
| Q(Sr)/e | Q(Fe)/e | Q(N)/e | Q(O)/e | Q(Mn)/e | ΔQNO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-NO | — | — | 0.112 | 0.135 | –1.636 | 0.245 |
| LaSrMnO3-NO | –1.664 | — | 0.106 | 0.126 | –1.634 | 0.226 |
| LaMnFeO3-NO | — | –1.601 | 0.110 | 0.146 | –1.641 | 0.256 |
| LaSrMnFeO3-NO | –1.658 | –1.591 | 0.148 | 0.101 | –1.698 | 0.249 |



| [1] |
doi: 10.6023/A19040129 |
|
(武卓敏, 石勇, 李春艳, 牛丹阳, 楚奇, 熊巍, 李新勇, 化学学报, 2019, 77, 758.)
doi: 10.6023/A19040129 |
|
| [2] |
doi: 10.6023/A20100478 |
|
(张雅祺, 楚奇, 石勇, 高金索, 熊巍, 黄磊, 丁越, 化学学报, 2021, 79, 361.)
doi: 10.6023/A20100478 |
|
| [3] |
doi: 10.1016/j.jcat.2024.115925 |
| [4] |
doi: 10.1016/j.jece.2024.113593 |
| [5] |
doi: 10.1016/j.joei.2022.07.006 |
| [6] |
doi: 10.1016/j.jece.2025.115568 |
| [7] |
doi: 10.1016/j.fuproc.2021.106798 |
| [8] |
|
|
(赵明新, 洪嘉奇, 王莹, 齐瑞杰, 刘晓刚, 赵田田, 王虹, 低碳化学与化工, 2023, 48, 85.)
|
|
| [9] |
doi: 10.3390/en16227609 |
| [10] |
doi: 10.1016/j.apcatb.2024.124846 |
| [11] |
doi: 10.1016/j.apcata.2023.119231 |
| [12] |
doi: 10.1016/j.cej.2019.04.178 |
| [13] |
doi: 10.1016/j.jece.2021.106612 |
| [14] |
doi: 10.1016/j.molcata.2006.05.008 |
| [15] |
doi: 10.1016/j.jre.2020.12.017 |
| [16] |
doi: 10.1016/j.seppur.2024.127272 |
| [17] |
doi: 10.1016/j.apsusc.2019.145158 |
| [18] |
doi: 10.1007/s10562-016-1860-0 |
| [19] |
doi: 10.1021/acs.iecr.9b01088 |
| [20] |
doi: 10.1039/C9TA09764C |
| [21] |
doi: 10.1016/j.fuel.2024.133891 |
| [22] |
doi: 10.1103/PhysRevB.88.174426 |
| [23] |
|
|
(董抒华, 田贵山, 冯柳, 中国有色金属学报, 2008, 1353.)
|
|
| [24] |
doi: 10.1038/s41929-021-00656-4 |
| [25] |
doi: 10.1016/j.apsusc.2021.151234 |
| [26] |
doi: 10.1016/j.apsusc.2025.163057 |
| [27] |
doi: 10.1016/j.jhazmat.2020.123576 |
| [28] |
doi: 10.1016/0021-9797(87)90248-7 |
| [29] |
doi: 10.1016/S0920-5861(00)00300-X |
| [30] |
|
|
(徐鲁华, 吴晓东, 陈尊, 翁端, 中国稀土学报, 2002, (4), 378.)
|
|
| [31] |
doi: 10.1016/j.cej.2023.141814 |
| [32] |
doi: 10.1016/j.envres.2023.118037 |
| [33] |
doi: 10.1016/j.apcatb.2015.09.002 |
| [34] |
|
| [35] |
doi: 10.1016/j.apcatb.2025.125066 |
| [36] |
doi: 10.1016/S1872-2067(20)63666-X |
| [37] |
doi: 10.1021/acs.iecr.3c01971 |
| [38] |
doi: 10.1016/j.fuel.2019.02.113 |
| [39] |
|
| [1] | Yichun Lou, Chengli He, Linrui Wang, Xiaoli Cui. Mechanochemical Urea Synthesis Using Nitrogen, Water and Carbon Dioxide with TiO2 under Mild Conditions: An Experimental and Theoretical Study [J]. Acta Chimica Sinica, 2026, 84(1): 73-85. |
| [2] | Shoufei Cheng, Jing Li, Lin Ling, Yuxue Li, Long Lu. Density Functional Theory Research on Decomposition Mechanisms of Nitroglycerin [J]. Acta Chimica Sinica, 2025, 83(8): 833-843. |
| [3] | Zhengbing Wei, Mengfan Bao, Shibiao Xu, Yi Cheng, Shijie Chen, Na Lin, Aiqin Mao. Fluorine Doping-Induced Intrinsic Defect Modulation in Rock-Salt-Type High-Entropy Oxide for Lithium Storage Performance Optimization [J]. Acta Chimica Sinica, 2025, 83(8): 868-877. |
| [4] | Meng-Xin Xu, Zhi-Jian Yang, Jing Sun, Wen-Qiang Zou, Zhong-Ning Xu, Guo-Cong Guo. Oxygen Vacancies in Pd/Pr-CeO2 Catalyst Regulate the Selectivity of CO Esterification to Dimethyl Carbonate [J]. Acta Chimica Sinica, 2025, 83(7): 655-660. |
| [5] | Nana Zhang, Jing Li. Oxygen Vacancies Enhance the Activity of PdNi/HfO2 Catalysts for Ethylene Glycol Electrocatalytic Oxidation [J]. Acta Chimica Sinica, 2025, 83(7): 709-715. |
| [6] | Xin Wang, Yiwei Shi, Ruijie Yang, Zhiguo Song, Min Wang. Solvent-Free “One-Pot” Biginelli Reaction Catalyzed by Mononuclear Cd(II) Complex Containing Benzenesulfonic Acid Ligands [J]. Acta Chimica Sinica, 2025, 83(7): 674-684. |
| [7] | Yi-Lin Lu, Shengjie Dong, Fangchao Cui, Tingting Bo, Zhuo Mao. Theoretical Construction of Hittorf’s Violet Phosphorene/SnS2 van der Waals Heterojunction as Direct Photocatalyst for Overall Water Splitting [J]. Acta Chimica Sinica, 2025, 83(4): 377-389. |
| [8] | Wei Sun, Guoxiang Xin, Fei Liu, Teng Ju, Yutong Cheng, Jinling Song, Jinxiao Bao, Chaoke Bulin. Construction of Three-dimensional Graphene/oxygen-enriched Vacancy Fe2O3 Composites to Realize Ultra-high Energy Density of Supercapacitors [J]. Acta Chimica Sinica, 2025, 83(3): 256-265. |
| [9] | Minghui Chen, Boxin Zhang, Tao Wei, Zhaoxue Sun, Yaqing Feng, Bao Zhang. Theoretical Calculation Studies on Interlayer Displacement Behavior and Photogenerated Carrier Dynamics of Covalent Triazine Frameworks [J]. Acta Chimica Sinica, 2025, 83(2): 93-100. |
| [10] | Naixin Zhang, Weiqun Shi, Congzhi Wang. Theoretical Studies of Divalent Actinide Complexes AnB8 [J]. Acta Chimica Sinica, 2025, 83(12): 1523-1529. |
| [11] | Ying Ma, Weixi Chen, Yuchen Liu, Ziyi Liu, Tao Wu, An-Hui Lu, Dongqi Wang. Density Functional Theory Study of Hexagonal Boron Nitride Oxidation Mode [J]. Acta Chimica Sinica, 2025, 83(1): 52-59. |
| [12] | Fanfan Zhang, Yuantao Cai, Jianbo Tao, Guoju Chang, Xinchen Guo, Shiyou Hao. Effect of Zn, C Introduction Amount and Calcination Temperature on the Photocatalytic Reduction of Cu2+over ZnO/C/CeO2 [J]. Acta Chimica Sinica, 2024, 82(8): 871-878. |
| [13] | Yuqing Zhao, Dong Liang, Jihui Jia, Rongmin Yu, Can-Zhong Lu. Synthesis and Characterization of an Emissive Ag(I) Complex with a D-A Type Ligand Containing Two Electron-withdrawing Groups [J]. Acta Chimica Sinica, 2024, 82(5): 486-492. |
| [14] | Guojing Wang, Yonghui Chen, Xiuqin Zhang, Junsheng Zhang, Junmin Xu, Jing Wang. Magnetic and Photoelectrocatalytic Properties of BiVO4 Surface Heterojunctions Controlled by Oxygen Vacancies [J]. Acta Chimica Sinica, 2024, 82(4): 409-415. |
| [15] | Yu-Qiang Zhao, Xia Zhang, Yunru Yang, Liping Zhu, Ying Zhou. Design and Synthesis of Aggregation-Induced Emission Photocage Molecules for In Situ Photoactivation Imaging Studies [J]. Acta Chimica Sinica, 2024, 82(3): 265-273. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||