

DFT Mechanism of Cu Catalyzed Coupling Reaction to Alkyl Aryl Ethers
Received date: 2021-04-22
Online published: 2021-06-02
Supported by
National Natural Science Foundation of China(81773634)
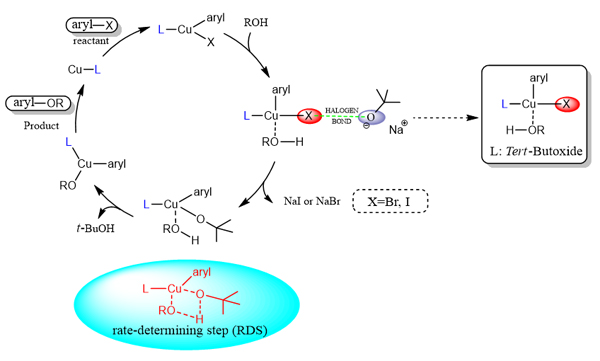
The mechanism of Cu catalyzed intramolecular Ullmann C―O coupling reaction of 2-(2-iodophenyl)ethan-1-ol was proposed and verified by density functional theory (DFT). All of the structures have been fully optimized using N,N-dimethylformamide as solvent by the Gaussian16 package with the B3LYP functional. In the process of optimization, the standard 6-31G (d) basis set was used for C, O, N and H atoms, while Stuttgart basis set (SDD) was used for Cu and I atoms. Grimme D3 method was used for dispersion correction of all calculations. The frequency calculation is carried out at the same theoretical level to determine whether the calculated geometry is minimum (zero imaginary frequency) or transition states (one imaginary frequency). The extra single-point calculations, including solvation and dispersion corrections, on the optimized geometries were employed to obtain improved Gibbs energy values with B3LYP/def2-tzvp in the continuum solvation model based on density (SMD). The intrinsic reaction coordinates (IRC) of all transition states were calculated to confirm that the structure connects the reactant and the product. The calculation shows that catalytic cycle starts from the in-situ formation of cuprous complex, and cuprous complex undergo oxidative addition to give Cu(III) complexes. The Cu(III) complex is coordinated with alcohol to form a four-coordinated Cu(III) complex. Then the substrate is activated by O-halogen bond, and the intermediates underwent ligand exchange to form another Cu(III) complexes, which transmits the desired alkyl aryl ethers and regenerates the catalyst cycle. Quantitative calculation shows that the reaction mechanism can be divided into four steps: (1) oxidative addition, (2) coordination effect, (3) ligand exchange, (4) reductive elimination. First, it was found that tert-butoxide was not only a base, but also a catalyst species. Secondly, it is pointed out that ligand exchange is the rate-determining step of the whole reaction. Before the ligand exchange, it is activated by a slowly approaching tert-butoxide to form a halogen bond (XB). The calculated results are in good agreement with the experimental data, which proves the rationality of the calculation mechanism and helps to explain the Ullmann reaction mechanism.
Qingmin Man , Zunyun Fu , Tiantian Liu , Mingyue Zheng , Hualiang Jiang . DFT Mechanism of Cu Catalyzed Coupling Reaction to Alkyl Aryl Ethers[J]. Acta Chimica Sinica, 2021 , 79(7) : 948 -952 . DOI: 10.6023/A21040172
| [1] | (a) Evano, G.; Wang, J. J.; Nitelet, A. Org. Chem. Front. 2017,2480. |
| [1] | (b) Ding, H.-W.; Li, J.; Guo, Q.-H.; Xiao, Y. Chinese J. Org. Chem. 2017, 37, 3112. (in Chinese) |
| [1] | (丁怀伟, 李娟, 郭庆辉, 肖琰, 有机化学, 2017, 37, 3112.) |
| [2] | (a) Liu, Y. J.; Zhang, D. M.; Xiao, S. H.; Qi, Y.; Liu, S. F. Asian J. Org. Chem. 2019, 8, 858. |
| [2] | (b) Merritt, J. M.; Andiappan, M.; Pietz, M. A.; Richey, R. N.; Sullivan, K. A.; Kjell, D. P. Org. Process Res. Dev. 2016, 20, 178. |
| [2] | (c) Monnier, F.; Taillefer, M. Angew. Chem. Int. Ed. 2009, 48, 6954. |
| [2] | (d) Cho, G. Y.; Remy, P.; Jansson, J.; Moessner, C.; Bolm, C. Org. Lett. 2004, 6, 3293. |
| [3] | Yang, J.-P.; Zhang, L.; Jin, X.-P.; Gao, H.-Q.; Fang, J.-H.; Li, R.-F.; Fang, Y.-W. Chinese J. Org. Chem. 2013, 33, 1647. (in Chinese) |
| [3] | (杨建平, 张莉, 金小平, 高浩其, 房江华, 李瑞丰, 方烨汶, 有机化学, 2013, 33, 1647.) |
| [4] | (a) Ouali, A.; Taillefer, M.; Spindler, J. F.; Jutand, A. Organometallics 2007, 26, 65. |
| [4] | (b) Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525. |
| [5] | Weingarten, H. J. Org. Chem. 1964, 29, 3624. |
| [6] | Paine, A. J. J. Am. Chem. Soc. 1987, 109, 1496. |
| [7] | Marcoux, J. F.; Doye, S.; Buchwald, S. L. J. Am. Chem. Soc. 1997, 119, 10539. |
| [8] | Mayoral, J. A.; Rodriguez-Rodriguez, S.; Salvatella, L. Chem. Eur. J. 2008, 14, 9274. |
| [9] | Tye, J. W.; Weng, Z.; Johns, A. M.; Incarvito, C. D.; Hartwig, J. F. J. Am. Chem. Soc. 2008, 130, 9971. |
| [10] | Yu, H. Z.; Jiang, Y. Y.; Fu, Y.; Liu, L. J. Am. Chem. Soc. 2010, 132, 18078. |
| [11] | Chen, Z. X.; Jiang, Y. W.; Zhang, L.; Guo, Y. L.; Ma, D. W. J. Am. Chem. Soc. 2019, 141, 3541. |
| [12] | Legon, A. C. Angew. Chem, Int. Ed. 1999, 38, 2687. |
| [13] | Lefevre, G.; Franc, G.; Adamo, C.; Jutand, A.; Ciofini, I. Organometallics 2012, 31, 914. |
| [14] | (a) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. |
| [14] | (b) Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Chem. Phys. Lett. 1989, 157, 200. |
| [14] | (c) Becke, A. D. J. Chem. Phys. 1993, 98, 5648. |
| [14] | (d) Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623. |
| [15] | (a) Hehre, W. J.; Ditchfield, R.; Pople, J. A. J. Chem. Phys. 1972, 56, 2257. |
| [15] | (b) Ditchfield, R.; Hehre, W. J.; Pople, J. A. J. Chem. Phys. 1971, 54, 724. |
| [16] | Bergner, A.; Dolg, M.; Kuchle, W.; Stoll, H.; Preuss, H. Mol. Phys. 1993, 80, 1431. |
| [17] | Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. J. Chem. Phys. 2010, 132, 154104. |
| [18] | Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378. |
| [19] | (a) Jover, J. Phys. Chem. Chem. Phys. 2017, 19, 29344. |
| [19] | (b) Jover, J. J. Chem. 2015, 2015, 430358. |
| [19] | (c) Jover, J.; Spuhler, P.; Zhao, L. G.; McArdle, C.; Maseras, F. Catal. Sci. Technol. 2014, 4, 4200. |
| [19] | (d) Jover, J.; Maseras, F. J. Org. Chem. 2014, 79, 11981. |
| [19] | (e) Jover, J. ACS Catal. 2014, 4, 4389. |
| [20] | (a) Fukui, K. J. Phys. Chem. 1970, 74, 4161. |
| [20] | (b) Fukui, K. Acc. Chem. Res. 1981, 14, 363. |
/
| 〈 |
|
〉 |