Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (9): 922-926.DOI: 10.6023/A19050158 Previous Articles Next Articles
Special Issue: 有机自由基化学
Article
汤娜娜a†, 邵鑫a†, 王明扬a, 吴新鑫a, 朱晨ab*( )
)
投稿日期:2019-05-01
发布日期:2019-06-04
通讯作者:
朱晨
E-mail:chzhu@suda.edu.cn
基金资助:
Tang, Nanaa†, Shao, Xina†, Wang, Mingyanga, Wu, Xinxina, Zhu, Chenab*( )
)
Received:2019-05-01
Published:2019-06-04
Contact:
Zhu, Chen
E-mail:chzhu@suda.edu.cn
Supported by:Share
Tang, Nana, Shao, Xin, Wang, Mingyang, Wu, Xinxin, Zhu, Chen. Sulfonyl Chlorides Mediated Alkynylation of Non-activated Alkenes via Distal Alkynyl Group Migration[J]. Acta Chimica Sinica, 2019, 77(9): 922-926.
| Entry | Photocatalyst (3 mol%) | Base (x equiv.) | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir{[dF(CF3)ppy]2-(dtbbpy)}PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 2 | Ir(ppy)2(dtbbpy)PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 3 | Ru(bpy)3Cl2?6H2O | Na2CO3 (1.0) | CH3CN | <5 |
| 4 | fac-Ir(ppy)3 | Na2CO3 (1.0) | CH3CN | 75 |
| 5 | fac-Ir(ppy)3 | Na2CO3 (0.8) | CH3CN | 57 |
| 6 | fac-Ir(ppy)3 | Na2CO3 (1.2) | CH3CN | 68 |
| 7 | fac-Ir(ppy)3 | Na2CO3 (1.0) | acetone | 68 |
| 8 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DCM | 58 |
| 9 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMSO | <10 |
| 10 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMF | <10 |
| Entry | Photocatalyst (3 mol%) | Base (x equiv.) | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir{[dF(CF3)ppy]2-(dtbbpy)}PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 2 | Ir(ppy)2(dtbbpy)PF6 | Na2CO3 (1.0) | CH3CN | <5 |
| 3 | Ru(bpy)3Cl2?6H2O | Na2CO3 (1.0) | CH3CN | <5 |
| 4 | fac-Ir(ppy)3 | Na2CO3 (1.0) | CH3CN | 75 |
| 5 | fac-Ir(ppy)3 | Na2CO3 (0.8) | CH3CN | 57 |
| 6 | fac-Ir(ppy)3 | Na2CO3 (1.2) | CH3CN | 68 |
| 7 | fac-Ir(ppy)3 | Na2CO3 (1.0) | acetone | 68 |
| 8 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DCM | 58 |
| 9 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMSO | <10 |
| 10 | fac-Ir(ppy)3 | Na2CO3 (1.0) | DMF | <10 |
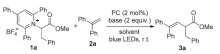
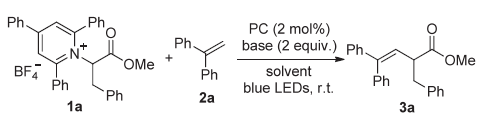
| Entry | CF3SO2Cl (x equiv.) | Photocatalyst (y mol%) | Base (z equiv.) | Solvent | Yield/% |
|---|---|---|---|---|---|
| 1 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | CH3CN | 29 |
| 2 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | acetone | 50 |
| 3 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | MeOH | 45 |
| 4 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMA | 33 |
| 5 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMSO | ND |
| 6 | 1.5 | fac-Ir(ppy)3 (3%) | Na2HPO4 (0.8) | acetone | 40 |
| 7 | 1.5 | fac-Ir(ppy)3 (3%) | Na2CO3 (0.8) | acetone | 36 |
| 8 | 1.5 | fac-Ir(ppy)3 (3%) | K2CO3 (0.8) | acetone | 42 |
| 9 | 1.5 | fac-Ir(ppy)3 (3%) | NaHCO3 (0.8) | acetone | 48 |
| 10 | 1.5 | fac-Ir(ppy)3 (3%) | DABCO (0.8) | acetone | 27 |
| 11 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.7) | acetone | 43 |
| 12 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.9) | acetone | 47 |
| 13 | 1.5 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 52 |
| 14 | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 54 |
| 15b | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 64 |
| 16b | 2.2 | Ru(bpy)3Cl2?6H2O (4%) | K2HPO4 (0.8) | acetone | ND |
| 17b | 2.2 | Ir(ppy)2(dtbbpy)PF6 (4%) | K2HPO4 (0.8) | acetone | ND |
| 18b | 2.2 | none | K2HPO4 (0.8) | acetone | ND |
| 19b,c | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | ND |
| Entry | CF3SO2Cl (x equiv.) | Photocatalyst (y mol%) | Base (z equiv.) | Solvent | Yield/% |
|---|---|---|---|---|---|
| 1 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | CH3CN | 29 |
| 2 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | acetone | 50 |
| 3 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | MeOH | 45 |
| 4 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMA | 33 |
| 5 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.8) | DMSO | ND |
| 6 | 1.5 | fac-Ir(ppy)3 (3%) | Na2HPO4 (0.8) | acetone | 40 |
| 7 | 1.5 | fac-Ir(ppy)3 (3%) | Na2CO3 (0.8) | acetone | 36 |
| 8 | 1.5 | fac-Ir(ppy)3 (3%) | K2CO3 (0.8) | acetone | 42 |
| 9 | 1.5 | fac-Ir(ppy)3 (3%) | NaHCO3 (0.8) | acetone | 48 |
| 10 | 1.5 | fac-Ir(ppy)3 (3%) | DABCO (0.8) | acetone | 27 |
| 11 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.7) | acetone | 43 |
| 12 | 1.5 | fac-Ir(ppy)3 (3%) | K2HPO4 (0.9) | acetone | 47 |
| 13 | 1.5 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 52 |
| 14 | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 54 |
| 15b | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | 64 |
| 16b | 2.2 | Ru(bpy)3Cl2?6H2O (4%) | K2HPO4 (0.8) | acetone | ND |
| 17b | 2.2 | Ir(ppy)2(dtbbpy)PF6 (4%) | K2HPO4 (0.8) | acetone | ND |
| 18b | 2.2 | none | K2HPO4 (0.8) | acetone | ND |
| 19b,c | 2.2 | fac-Ir(ppy)3 (4%) | K2HPO4 (0.8) | acetone | ND |


| [1] |
For selected reviews: (a) Chen, F.; Wang, T.; Jiao, N . Chem. Re. 2014, 114, 8613;
doi: 10.6023/cjoc201803053 |
|
(b) Souillart, L.; Cramer, N . Chem. Re. 2015, 115, 9410;
doi: 10.6023/cjoc201803053 |
|
|
(c) Song, F.; Gou, T.; Wang, B.-Q.; Shi, Z.-J . Chem. Soc. Rev. 2018, 47, 7078;
doi: 10.6023/cjoc201803053 |
|
|
(d) Wu, X.; Zhu, C . Chem. Rec. 2018, 18, 587;
doi: 10.6023/cjoc201803053 |
|
|
(e) Wu, X.; Zhu, C . Chin. J. Chem. 2019, 37, 171;
doi: 10.6023/cjoc201803053 |
|
|
(f) Wang, B.; Liu, Y.; Hao, Z.; Hou, J.; Li, J.; Li, S.; Pan, S.; Zeng, M.; Wang, Z . Chin. J. Org. Chem. 2018, 38, 1872 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳 , 有机化学, 2018, 38, 1872.)
doi: 10.6023/cjoc201803053 |
|
|
(g) Liang, T.; Jiang, L.; Gan, M.; Su, X.; Li, Z . Chin. J. Org. Chem. 2017, 37, 3096 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 梁婷婷, 姜岚, 干苗苗, 苏鑫, 李争宁 , 有机化学, 2017, 37, 3096.)
doi: 10.6023/cjoc201803053 |
|
|
(h) Wu, K.; Song, C.; Cui, D . Chin. J. Org. Chem. 2017, 37, 586 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 吴空, 宋婵, 崔冬梅 , 有机化学, 2017, 37, 586.)
doi: 10.6023/cjoc201803053 |
|
|
(i) Zhong, J.-J.; Meng, Q.-Y.; Chen, B., Tung, C.-H.; Wu, L.-Z . Acta Chim. Sinica. 2017, 75, 34 (in Chinese).
doi: 10.6023/cjoc201803053 |
|
|
( 钟建基, 孟庆元, 陈彬, 佟振合, 吴骊珠 , 化学学报, 2017, 75, 34.)
doi: 10.6023/cjoc201803053 |
|
| [2] |
For selected reviews on FGM.(a) Wu, X.; Wu, S.; Zhu, C . Tetrahedron Let. 2018, 59, 1328;
doi: 10.6023/A18080313 |
|
(b) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C . Chem. Soc. Re. 2018, 47, 654;
doi: 10.6023/A18080313 |
|
|
For selected examples from our group, see: (c) Wu, Z.; Ren, R.; Zhu, C.; . Angew. Chem. Int. Ed. 2016, 55, 10821;
doi: 10.6023/A18080313 |
|
|
(d) Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C . J. Am. Chem. Soc. 2017, 139, 1388;
doi: 10.6023/A18080313 |
|
|
(e) Ren, R.; Wu, Z.; Huan, L.; Zhu, C . Adv. Synth. Catal. 2017, 359, 3052;
doi: 10.6023/A18080313 |
|
|
(f) Ji, M.; Wu, Z.; Yu, J.; Wan, X.; Zhu, C . Adv. Synth. Catal. 2017, 359, 1959;
doi: 10.6023/A18080313 |
|
|
(g) Wang, M.; Wu, Z.; Zhang, B.; Zhu, C . Org. Chem. Front. 2018, 5, 1896;
doi: 10.6023/A18080313 |
|
|
(h) Yu, J.; Wang, D.; Xu, Y.; Wu, Z.; Zhu, C . Adv. Synth. Catal. 2018, 360, 744;
doi: 10.6023/A18080313 |
|
|
(i) Zhang, H.; Wu, X.; Zhao, Q.; Zhu, C . Chem. Asian J. 2018, 13, 2453;
doi: 10.6023/A18080313 |
|
|
(j) Chen, D.; Wu, Z.; Yao, Y.; Zhu, C . Org. Chem. Front. 2018, 5, 2370;
doi: 10.6023/A18080313 |
|
|
(k) Tang, N.; Yang, S.; Wu, X.; Zhu, C . Tetrahedron. 2019, 75, 1639;
doi: 10.6023/A18080313 |
|
|
(l) Chen, D.; Ji, M.; Yao, Y.; Zhu, C . Acta Chim. Sinica. 2018, 76, 951 (in Chinese).
doi: 10.6023/A18080313 |
|
|
( 陈栋, 吉梅山, 姚英明, 朱晨 , 化学学报, 2018, 76, 951.)
doi: 10.6023/A18080313 |
|
|
(m) Ji, M.; Wu, Z.; Zhu, C . Chem. Commun. 2019, 55, 2368.
doi: 10.6023/A18080313 |
|
| [3] |
For selected examples of remote FGM from other groups.(a) Thaharn, W.; Soorukram, D.; Kuhakarn, C.; Tuchinda, P.; Reutrakul, V.; Pohmakotr, M . Angew. Chem. Int. E. 2014, 53, 2212;
doi: 10.1002/anie.201310747 |
|
(b) Li, L.; Li, Z.-L.; Wang, F.-L.; Guo, Z.; Cheng, Y.-F.; Wang, N.; Dong, X.-W.; Fang, C.; Liu, J.; Hou, C.; Tan, B.; Liu, X.-Y. . Nat. Commu. 2016, 7, 13852;
doi: 10.1002/anie.201310747 |
|
|
(c) Li, Z.-L.; Li, X.-H.; Wang, N.; Yang, N.-Y.; Liu, X.-Y . Angew. Chem. Int. Ed. 2016, 55, 15100;
doi: 10.1002/anie.201310747 |
|
|
(d) Li, L.; Li, Z.-L.; Gu, Q.-S.; Wang, N.; Liu, X.-Y . Sci. Adv. 2017, 3, e1701487;
doi: 10.1002/anie.201310747 |
|
|
(e) Wang, N.; Li, L.; Li, Z.-L.; Yang, N.-Y.; Guo, Z.; Zhang, H.-X.; Liu, X.-Y . Org. Lett. 2016, 18, 6026;
doi: 10.1002/anie.201310747 |
|
|
(f) Tang, X.; Studer, A . Angew. Chem. Int. Ed. 2018, 57, 814;
doi: 10.1002/anie.201310747 |
|
|
(g) Wang, H.; Xu, Q.; Yu, S . Org. Chem. Front. 2018, 5, 2224;
doi: 10.1002/anie.201310747 |
|
|
(h) He, Y.; Wang, Y.; Gao, J.; Zeng, L.; Li, S.; Wang, W.; Zheng, X.; Zhang, S.; Gu, L.; Li, G . Chem. Commun. 2018, 54, 7499;
doi: 10.1002/anie.201310747 |
|
|
(i) Gu, L.; Gao, Y.; Ai, X.; Jin, C.; He, Y.; Li, G.; Yuan, M . Chem. Commun. 2017, 53, 12946;
doi: 10.1002/anie.201310747 |
|
|
(j) Wei, X.-J.; Noël, T . J. Org. Chem. 2018, 83, 11377.
doi: 10.1002/anie.201310747 |
|
| [4] |
Xu, Y.; Wu, Z.; Jiang, J.; Ke, Z.; Zhu, C . Angew. Chem. Int. E. 2017, 56, 4545.
doi: 10.1002/anie.201700413 |
| [5] | For selected examples of alkynyl migration.(a) Tang, X.; Studer, A . Chem. Sc. 2017, 8, 6888 |
| (b) Liu, J.; Li, W.; Xie, J.; Zhu, C . Org. Chem. Fron. 2018, 5, 797; | |
| (c) Zhao, Q.; Ji, X.-S.; Gao, Y.-Y.; Hao, W.-J.; Zhang, K.-Y.; Tu, S.-J.; Jiang, B . Org. Lett. 2018, 2. 3596. | |
| [6] |
(a) Meadows, D. C.; Gervay-Hague, J . Med. Res. Re. 2006, 26, 793;
doi: 10.1039/C6OB02424F |
|
(b) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X . Curr. Top. Med. Che. 2016, 16, 1200;
doi: 10.1039/C6OB02424F |
|
|
(c) Shaaban, S.; Liang, S.; Liu, N.-W.; Manolikakes, G . Org. Biomol. Chem. 2017, 15, 1947;
doi: 10.1039/C6OB02424F |
|
|
(d) Qiu, G.; Zhou, K.; Gao, L.; Wu, J . Org. Chem. Front. 2018, 5, 691.
doi: 10.1039/C6OB02424F |
| [1] | Guanglong Huang, Xiao-Song Xue. Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source [J]. Acta Chimica Sinica, 2024, 82(2): 132-137. |
| [2] | Fei Li, Huili Ding, Chaozhong Li. Hydrotrifluoromethylation of Alkenes with a Fluoroform-Derived Trifluoromethylboron Complex [J]. Acta Chimica Sinica, 2023, 81(6): 577-581. |
| [3] | Zhao, Qi, Tu, Shu-Jiang, Jiang, Bo. Hydrogen Radical Initiated 1,2-Alkynyl Migration [J]. Acta Chimica Sinica, 2019, 77(9): 927-931. |
| [4] | Chen Dong, Ji Meishan, Yao Yingming, Zhu Chen. Difunctionalization of Unactivated Alkenes through SCF3 Radical-triggered Distal Functional Group Migration [J]. Acta Chimica Sinica, 2018, 76(12): 951-955. |
| [5] | Rong Jian, Ni Chuanfa, Wang Yunze, Kuang Cuiwen, Gu Yucheng, Hu Jinbo. Radical Fluoroalkylation of Aryl Alkenes with Fluorinated Sulfones by Visible-Light Photoredox Catalysis [J]. Acta Chimica Sinica, 2017, 75(1): 105-109. |
| [6] | Gou Baoquan, Yang Chao, Zhang Lei, Xia Wujiong. Visible-Light Induced Trifluoromethylation of Internal Olefinic C-H Bonds through Photoredox Catalysis [J]. Acta Chim. Sinica, 2017, 75(1): 66-69. |
| [7] | Pan Fei, Shi Zhangjie. Transition Metal-Catalyzed C—H Trifluoromethylation [J]. Acta Chimica Sinica, 2012, 70(16): 1679-1681. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||