有机化学 ›› 2012, Vol. ›› Issue (03): 621-623.DOI: 10.6023/cjoc1107061 上一篇 下一篇
研究简报
张红梅, 曹湖军, 张志勇, 彭娟娟, 崔宏宇, 张天静, 雷钢铁
Zhang Hongmei, Cao Hujun, Zhang Zhiyong, Peng Juanjuan, Cui Hongyu, Zhang Tianjing, Lei Gangtie
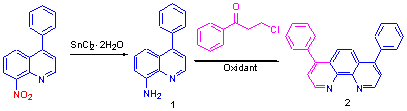
以4-苯基-8-硝基喹啉为起始原料, 经还原得到4-苯基-8-氨基喹啉, 再以I2/KI 为氧化剂, 在乙酸和盐酸的存在下, 用Skraup 法合成了4,7-二苯基-1,10-菲啰啉. 化合物结构经IR 和1 H NMR 得到了证实. 实验研究得到了最佳的合成条件为: n(3-氯苯丙酮)∶n(4-苯基-8-氨基喹啉)=1.5∶1, I2/KI 用量为8%, 反应温度120 ℃, 反应时间2.5 h. 产品收率可达82%.