有机化学 ›› 2021, Vol. 41 ›› Issue (7): 2587-2600.DOI: 10.6023/cjoc202102014 上一篇 下一篇
综述与进展
张永红a,b,*( ), 唐承宗a, 刘永红a, 刘晨江a,b,*(
), 唐承宗a, 刘永红a, 刘晨江a,b,*( )
)
收稿日期:2021-02-02
修回日期:2021-03-23
发布日期:2021-04-16
通讯作者:
张永红, 刘晨江
基金资助:
Yonghong Zhanga,b( ), Chengzong Tanga, Yonghong Liua, Chenjiang Liua,b(
), Chengzong Tanga, Yonghong Liua, Chenjiang Liua,b( )
)
Received:2021-02-02
Revised:2021-03-23
Published:2021-04-16
Contact:
Yonghong Zhang, Chenjiang Liu
Supported by:文章分享

芳基三氮烯因制备简单, 稳定性好, 反应位点多, 反应条件温和等优点, 作为一种稳定的芳基重氮盐替代物被广泛用于有机合成中. 主要综述了近年来以芳基三氮烯作为芳基前体和芳基偶氮前体在有机合成中的应用研究进展.
张永红, 唐承宗, 刘永红, 刘晨江. 芳基三氮烯作为芳基前体和芳基偶氮前体在有机合成中的应用研究进展[J]. 有机化学, 2021, 41(7): 2587-2600.
Yonghong Zhang, Chengzong Tang, Yonghong Liu, Chenjiang Liu. Research Progress of Aryltriazene as Aryl Precursor and Aryl-Azo Precursors in Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2587-2600.
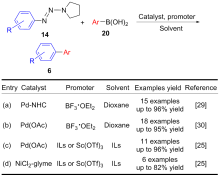
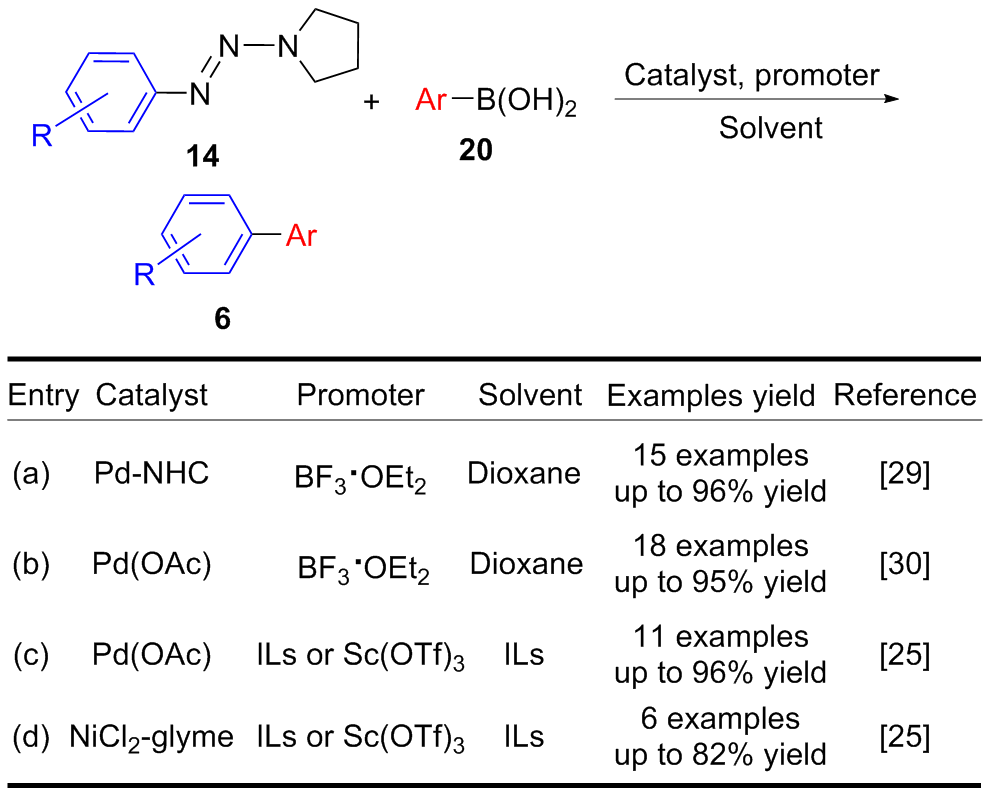

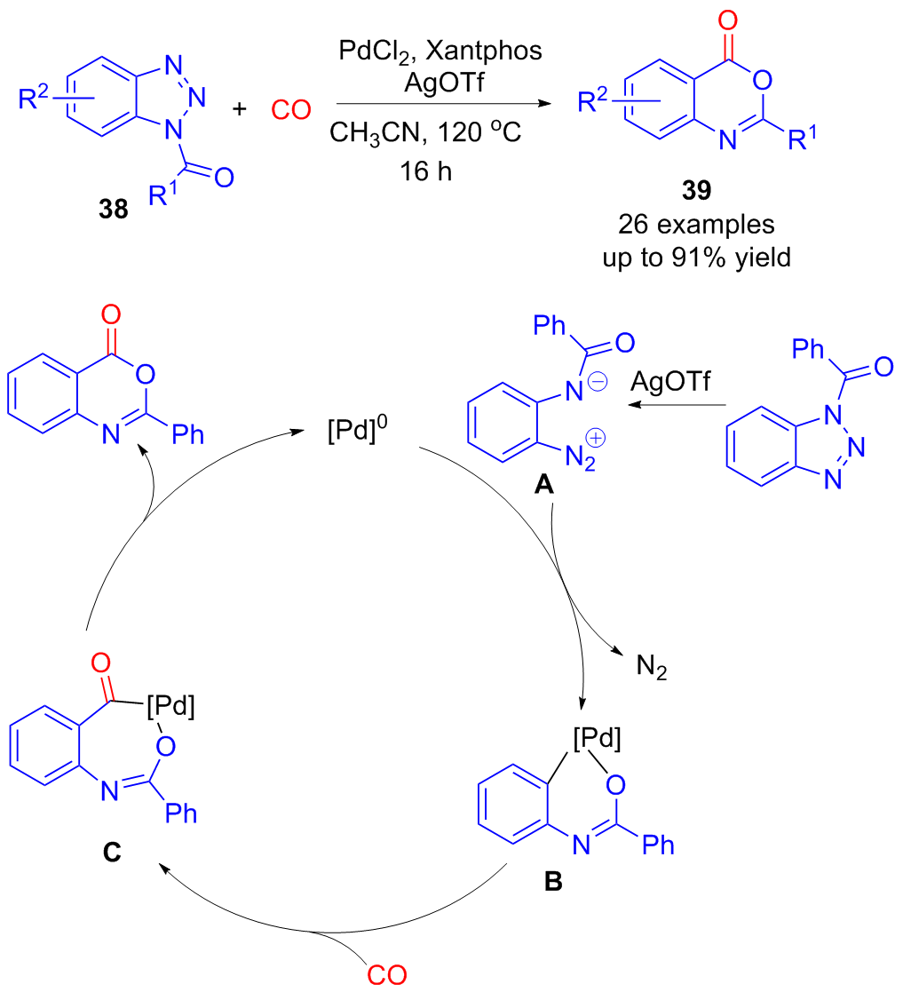
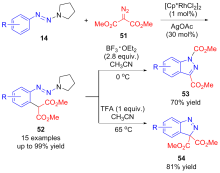
| [1] |
(a) Rouzer,C. A.; Sabourin, M.; Skinner,T. L.; Thompson,E. J.; Wood,T. O.; Chmurny,G. N.; Klose,J. R.; Roman,J. M.; Smith,R. H.; Michejda,C. J. Chem. Res. Toxicol. 1996, 9,172.
doi: 10.1021/tx9500639 |
|
(b) Gross,M. L.; Blank,D. H.; Welch,W. M. J. Org. Chem. 1993, 58,2104.
doi: 10.1021/jo00060a028 |
|
| [2] |
Griess, P. Justus Liebigs Ann. Chem. 1862, 121,258.
|
| [3] |
Connors,T. A.; Goddard,P. M.; Merai, K.; Ross,W. C.J.; Wilman,D. E.V. Biochem. Pharmacol. 1976, 25,241.
doi: 10.1016/0006-2952(76)90207-0 |
| [4] |
Li, Q.; Jin, C.; Petukhov,P. A.; Rukavishnikov,A. V.; Zaikova,T. O.; Phadke, A.; LaMunyon,D. H.; Lee,M. D.; Keana,J. F.W. J. Org. Chem. 2004, 69,1010.
doi: 10.1021/jo035640+ |
| [5] |
Romanato, P.; Duttwyler, S.; Linden, A.; Baldridge,K. K.; Siegel,J. S. J. Am. Chem. Soc. 2010, 132,7828.
doi: 10.1021/ja9109665 |
| [6] |
Jenny,N. M.; Wang, H.; Neuburger, M.; Fuchs, H.; Chi, L.; Mayor, M. Eur. J. Org. Chem. 2012, 14,2738.
|
| [7] |
Romanato, P.; Duttwyler, S.; Linden, A.; Baldridge,K. K.; Siegel,J. S. J. Am. Chem. Soc. 2011, 133,11844.
doi: 10.1021/ja2040392 |
| [8] |
Kirk,M. L.; Shultz,D. A.; Stasiw,D. E.; Lewis,G. F.; Wang, G.; Brannen,C. L.; Sommer,R. D.; Boyle,P. D. J. Am. Chem. Soc. 2013, 135,17144.
doi: 10.1021/ja4081887 |
| [9] |
Romanato, P.; Duttwyler, S.; Linden, A.; Baldridge,K. K.; Siegel,J. S. J. Phys. Org. Chem. 2014, 27,277.
doi: 10.1002/poc.3265 |
| [10] |
Xue, D.; Luo,S. -P.; Zhan,S. -Z. New J. Chem. 2017, 41,8503.
doi: 10.1039/C7NJ01705G |
| [11] |
Kimball,D. B.; Haley,M. M. Angew. Chem. Int. Ed. 2002, 41,3338.
doi: 10.1002/1521-3773(20020916)41:18【-逻*辑*与-】#x00026;lt;3338::AID-ANIE3338【-逻*辑*与-】#x00026;gt;3.0.CO;2-7 |
| [12] |
(a) Zhang, Y.; Cao, D.; Liu, W.; Hu, H.; Zhang, X.; Liu, C. Curr. Org. Chem. 2015, 19,151.
doi: 10.2174/1385272819666150119222344 |
|
(b) Botting,N. P.; Challis,B. C. J. Chem. Soc. Chem. Commun. 1989,1585.
|
|
|
(c) Lazny, R.; Poplawski, J.; Köbberling, J.; Enders, D.; Bräse, S. Synlett 1999,1304.
|
|
| [13] |
(a) Sousa, A.; Santos, F.; Gaspar,M. M.; Calado, S.; Pereira,J. D.; Mendes, E.; Francisco,A. P.; Perry,M. J. Bioorg. Med. Chem. 2017, 25,3900.
|
|
(b) Mouhri,Z. S.; Goodfellow, E.; Kelley,S. P.; Stein,R. S.; Rogers,R. D.; Jean-Claude,B. J. Molecules 2017, 22,1183.
doi: 10.3390/molecules22071183 |
|
| [14] |
Wang, Q.; Wang,C. -B.; Pang,F. -Q.; Lu, T.; Yin,H. -Q.; Chen,F. -X. Chin. Chem. Lett. 2017, 28,1784.
doi: 10.1016/j.cclet.2017.04.006 |
| [15] |
(a) Wang, Q.; Pang, F.; Wang, G.; Huang, J.; Nie, F.; Chen, F. Chem. Commun. 2017, 53,2327.
doi: 10.1039/C6CC08179G |
|
(b) Khramov,D. M.; Bielawski,C. W. J. Org. Chem. 2007, 72,9407.
doi: 10.1021/jo070789x |
|
| [16] |
(a) Sieh,D. H.; Wilbur,D. J.; Michejda,C. J. J. Am. Chem. Soc. 1980, 102,3883.
doi: 10.1021/ja00531a033 |
|
(b) Nakhai, A.; Stensland, B.; Svensson,P. H.; Bergman, J. Eur. J. Org. Chem. 2010, 34,6588.
|
|
|
(c) Suleymanov,A. A.; Scopelliti, R.; Tirani,F. F.; Severin, k. Org. Lett. 2018, 20,3323.
doi: 10.1021/acs.orglett.8b01214 |
|
| [17] |
Kiefer, G.; Riedel, T.; Dyson,P. J.; Scopelliti, R.; Severin, K. Angew. Chem. Int. Ed. 2015, 54,302.
doi: 10.1002/anie.201408597 |
| [18] |
(a) Tan,J. -F.; Bormann,C. T.; Perrin,F. G.; Chadwick,F. M.; Severin, K.; Cramer, N. J. Am. Chem. Soc. 2019, 141,10372.
doi: 10.1021/jacs.9b04111 |
|
(b) Wezeman, T.; Scopelliti, R.; Tirani,F. F.; Severin, K. Adv. Synth. Catal. 2019, 361,1383.
doi: 10.1002/adsc.v361.6 |
|
| [19] |
(a) Fanghänel, E.; Poleschner, H.; Radelgia, R.; Hänsel, R. J.Prakt. Chem. 1977, 319,813.
doi: 10.1002/prac.19773190518 |
|
(b) Nguyen,M. T.; Hoesch, L. Helv. Chim. Acta 1986, 69,1627.
doi: 10.1002/(ISSN)1522-2675 |
|
|
(c) Schmiedekamp, A.; Smith,R. H.; Michejda,C. J. J. Org. Chem. 1988, 53,3433.
doi: 10.1021/jo00250a006 |
|
|
(d) Fanghänel E.; Ortmann W. J. Prakt. Chem. 1989, 331,721.
doi: 10.1002/prac.19893310503 |
|
|
(e) Ozment,J. L.; Schmiedekamp,A. M.; Schultz-Merkel,L. A.; Smith,R. H.; Michejda,C. J. J. Am. Chem. Soc. 1991, 113,397.
doi: 10.1021/ja00002a003 |
|
|
(f) Schmiedekamp,A. M.; Topol,I. A.; Burt,S. K.; Razafinjanahary, H.; Chermette, H.; Pfaltzgraff, T.; Michejda,C. J. J. Comput. Chem. 1994, 15,875.
doi: 10.1002/(ISSN)1096-987X |
|
|
(g) Rakotondradany, F.; Williams,C. I.; Whitehead,M. A.; Jean-Claude,B. J. J. Mol. Struct.:THEOCHEM 2001, 535,217.
doi: 10.1016/S0166-1280(00)00595-9 |
|
|
(h) Kimani,F. W.; Jewett,J. C. Angew. Chem. Int. Ed. 2015, 54,4051.
doi: 10.1002/anie.201411277 |
|
| [20] |
Landman,I. R.; Suleymanov,A. A.; Fadaei-Tirani, F.; Scopelliti, R.; Chadwick,F. M.; Severin, K. Dalton Trans. 2020, 49,2317.
doi: 10.1039/D0DT00049C |
| [21] |
Bhattacharya, S.; Majee, S.; Mukherjee, R.; Sengupta, S. Synth. Commun. 1995, 25,651.
doi: 10.1080/00397919508011402 |
| [22] |
Sengupta, S.; Sadhukkan,S. K. Tetrahedron Lett. 1998, 39,715.
doi: 10.1016/S0040-4039(97)10643-8 |
| [23] |
Bräse, S.; Schroen, M. Angew. Chem. Int. Ed. 1999, 38,1071.
|
| [24] |
Kumar, S.; Pandey,A. K.; Singh, R.; Singh,K. N. Eur. J. Org. Chem. 2018, 43,5942.
|
| [25] |
Sutar,S. M.; Savanur,H. M.; Malunavar,S. S.; Prabhala, P.; Kalkhambkar,R. G.; Laali,K. K. Eur. J. Org. Chem. 2019,6088.
|
| [26] |
Vishwakarma,R. K.; Kumar, S.; Sharma,A. K.; Singh, R.; Singh,K. N. ChemistrySelect 2019, 4,4064.
doi: 10.1002/slct.v4.14 |
| [27] |
Saeki, T.; Son,E. C.; Tamao, K. Org. Lett. 2004, 6,617.
doi: 10.1021/ol036436b |
| [28] |
Liu,C. -Y.; Gavryushin, A.; Knochel, P. Chem. Asian J. 2007, 2,1020.
doi: 10.1002/(ISSN)1861-471X |
| [29] |
Nan, G.; Ren F.; Luo, M. Beilstein J. Org. Chem. 2010, 6, No.70. doi: 10.3762/bjoc.6.70.
doi: 10.3762/bjoc.6.70 |
| [30] |
Nan, G.; Zhu, F.; Wei, Z. Chin. J. Chem. 2011, 29,72.
doi: 10.1002/cjoc.v29.1 |
| [31] |
Nan, G..; Zhou, J. Chin. J. Org. Chem. 2012, 32,1695 (in Chinese).
doi: 10.6023/cjoc1201311 |
|
( 南光明, 周均, 有机化学, 2012, 32,1695.)
|
|
| [32] |
Nan,G. M.; Zhou, J.; Yan,W. L. Asian J. Chem. 2013, 25,10322.
doi: 10.14233/ajchem |
| [33] |
Mao, S.; Chen, Z.; Wang, L.; Khadka, D.; Xin, M.; Li, P.; Zhang, S. J. Org. Chem. 2019, 84,463.
doi: 10.1021/acs.joc.8b02766 |
| [34] |
Sengupta, S.; Sadhukkan,S. K. Org. Synth. 2002, 79,52.
doi: 10.15227/orgsyn.079.0052 |
| [35] |
Saeki, T.; Matsunaga, T.; Son,E. C.; Tamao, K. Adv. Synth. Catal. 2004, 346,1689.
doi: 10.1002/adsc.200404212 |
| [36] |
Zhou, J.; Yang,W. J.; Wang,B. J.; Ren,H. J. Angew. Chem. Int. Ed. 2012, 51,12293.
doi: 10.1002/anie.v51.49 |
| [37] |
Xu, L.; Yang, W.; Zhang, L.; Miao, M.; Yang, Z.; Xu, X.; Ren, H. J. Org. Chem. 2014, 79,9206.
doi: 10.1021/jo501640d |
| [38] |
Liu, C.; Miao, T.; Zhang, L.; Li, P..; Zhang, Y.; Wang, L. Chem. Asian J. 2014, 9,2584.
doi: 10.1002/asia.201402274 |
| [39] |
Wang, R.; Falck,J. R. Org. Chem. Front. 2014, 1,1029.
doi: 10.1039/C4QO00213J |
| [40] |
Chaubey,N. R.; Vishwakarma,R. K.; Singh,K. N. ChemistrySelect 2019, 4,8522.
doi: 10.1002/slct.v4.29 |
| [41] |
Zificsak,C. -A.; Hlasta,D. -J. Tetrahedron 2004, 60,8991.
doi: 10.1016/j.tet.2004.07.016 |
| [42] |
Dai,W. C.; Wang,Z. X. Org. Chem. Front. 2017, 4,1281.
doi: 10.1039/C7QO00174F |
| [43] |
Liu, C.; Wang, Z.; Wang, L.; Li, P.; Zhang, Y. Org. Biomol. Chem. 2019, 17,9209.
doi: 10.1039/C9OB01883B |
| [44] |
Barragan, E.; Poyil,A. N.; Yang,C. -H.; Wang, H.; Bugarin, A. Org. Chem. Front. 2019, 6,152.
doi: 10.1039/C8QO00938D |
| [45] |
Li, W.; Wu,X. -F. Org. Biomol. Chem. 2015, 13,5090.
doi: 10.1039/C5OB00502G |
| [46] |
Li, W.; Wu,X. F. Org. Lett. 2015, 17,1910.
doi: 10.1021/acs.orglett.5b00603 |
| [47] |
Yin, Z.; Wang, Z.; Wu,X. -F. Eur. J. Org. Chem. 2017,3992.
|
| [48] |
Yin, Z.; Wang, Z.; Wu,X. -F. Org. Lett. 2017, 19,6232.
doi: 10.1021/acs.orglett.7b03184 |
| [49] |
Chand, S.; Kumar, S.; Singh, R.; Singh,K. N.; ChemistrySelect 2019, 4,718.
doi: 10.1002/slct.v4.2 |
| [50] |
Wippert,N. A.; Jung, N.; Bräse, S. ACS Comb. Sci. 2019, 21,568.
doi: 10.1021/acscombsci.9b00096 |
| [51] |
Barragan, E.; Noonikara-Poyil, A.; Bugarin, A. Asian J. Org. Chem. 2020, 9,593.
doi: 10.1002/ajoc.v9.4 |
| [52] |
Khazaei, A.; Kazem-Rostami, M.; Moosavi-Zare,A. R.; Bayat, M.; Saednia, S. Synlett 2012, 23,1893.
doi: 10.1055/s-0032-1316557 |
| [53] |
Zhang, Y.; Li, Y.; Zhang, X.; Jiang, X. Chem. Commun. 2015, 51,941.
doi: 10.1039/C4CC08367A |
| [54] |
Pandey,A. K.; Chand, S.; Singh, R.; Kumar, S.; Singh,K. N. ACS Omega 2020, 5,7627.
doi: 10.1021/acsomega.0c00472 |
| [55] |
Pandey,A. K.; Kumar, S.; Singh, R.; Singh,K. N. Tetrahedron 2018, 74,6704.
doi: 10.1016/j.tet.2018.09.059 |
| [56] |
Qi,X. -X.; Jiang,L. -B.; Zhou, C.; Peng,J. -B.; Wu,X. -F. ChemistryOpen 2017, 6,345.
doi: 10.1002/open.v6.3 |
| [57] |
Kimball,D. B.; Hayes,A. G.; Haley,M. M. Org. Lett. 2000, 2,3825.
doi: 10.1021/ol006517x |
| [58] |
(a) Kimball,D. B.; Herges, R.; Haley,M. M. J. Am. Chem. Soc. 2002, 124,1572.
doi: 10.1021/ja017227u |
|
(b) Kimball,D. B.; Weakley,T. J.R.; Haley,M. M. J. Org. Chem. 2002, 67,6395.
doi: 10.1021/jo020229s |
|
| [59] |
Zhu, C.; Yamane, M. Tetrahedron 2011, 67,4933.
doi: 10.1016/j.tet.2011.04.079 |
| [60] |
Yang, W.; Yang, Z.; Xu, L.; Zhang, L.; Xu, X.; Miao, M.; Ren, H. Angew. Chem. Int. Ed. 2013, 52,14135.
doi: 10.1002/anie.201306632 |
| [61] |
Wang, D.; Cui, S. Tetrahedron 2016, 72,2725.
doi: 10.1016/j.tet.2015.06.024 |
| [62] |
Cao, D.; Zhang, Y.; Liu, C.; Wang, B; Sun, Y.; Abdukadera, A.; Hu, H.; Liu, Q. Org. Lett. 2016, 18,2000.
doi: 10.1021/acs.orglett.6b00605 |
| [63] |
Zhang, Y.; Hu, H.; Liu, C.; Cao, D.; Wang, B.; Sun, Y.; Abdukader, A. Asian J. Org. Chem. 2017, 6,102.
doi: 10.1002/ajoc.v6.1 |
| [64] |
Zhang, Y.; Liu, Y.; Ma, X.; Ma, X.; Wang, B.; Li, H.; Huang, Y.; Liu, C. Dyes Pigm. 2018, 158,438.
doi: 10.1016/j.dyepig.2018.05.073 |
| [65] |
Liu, Y.; Ma, X.; Wu, G.; Liu, Z.; Yang, X.; Wang, B.; Liu, C.; Zhang, Y.; Huang, Y. New J. Chem. 2019, 43,9255.
doi: 10.1039/C9NJ01728C |
| [66] |
Liu, C.; Lv, J.; Luo,S. Z.; Cheng,J. P. Org. Lett. 2014, 16,5458.
doi: 10.1021/ol5027014 |
| [67] |
Sheng, M.; Frurip, D.; Gorman, D. J. Loss Prev. Process Ind. 2015, 38,114.
doi: 10.1016/j.jlp.2015.09.004 |
| [68] |
Firth,J. D.; Fairlamb,I. J.S. Org. Lett. 2020, 22,7057.
doi: 10.1021/acs.orglett.0c02685 |
| [1] | 南光明, 周均. Lewis酸诱导芳基三氮烯为底物Stille偶联反应研究[J]. 有机化学, 2012, 32(9): 1695-1699. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||