有机化学 ›› 2022, Vol. 42 ›› Issue (3): 714-731.DOI: 10.6023/cjoc202110011 上一篇 下一篇
综述与进展
收稿日期:2021-10-09
修回日期:2021-11-02
发布日期:2021-11-25
通讯作者:
贾铁争
基金资助:
Xue Li, Cong Wang, Tiezheng Jia( )
)
Received:2021-10-09
Revised:2021-11-02
Published:2021-11-25
Contact:
Tiezheng Jia
Supported by:文章分享
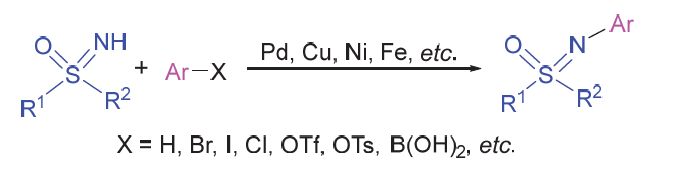
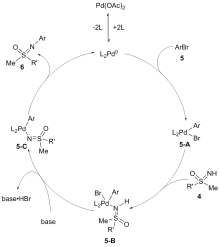
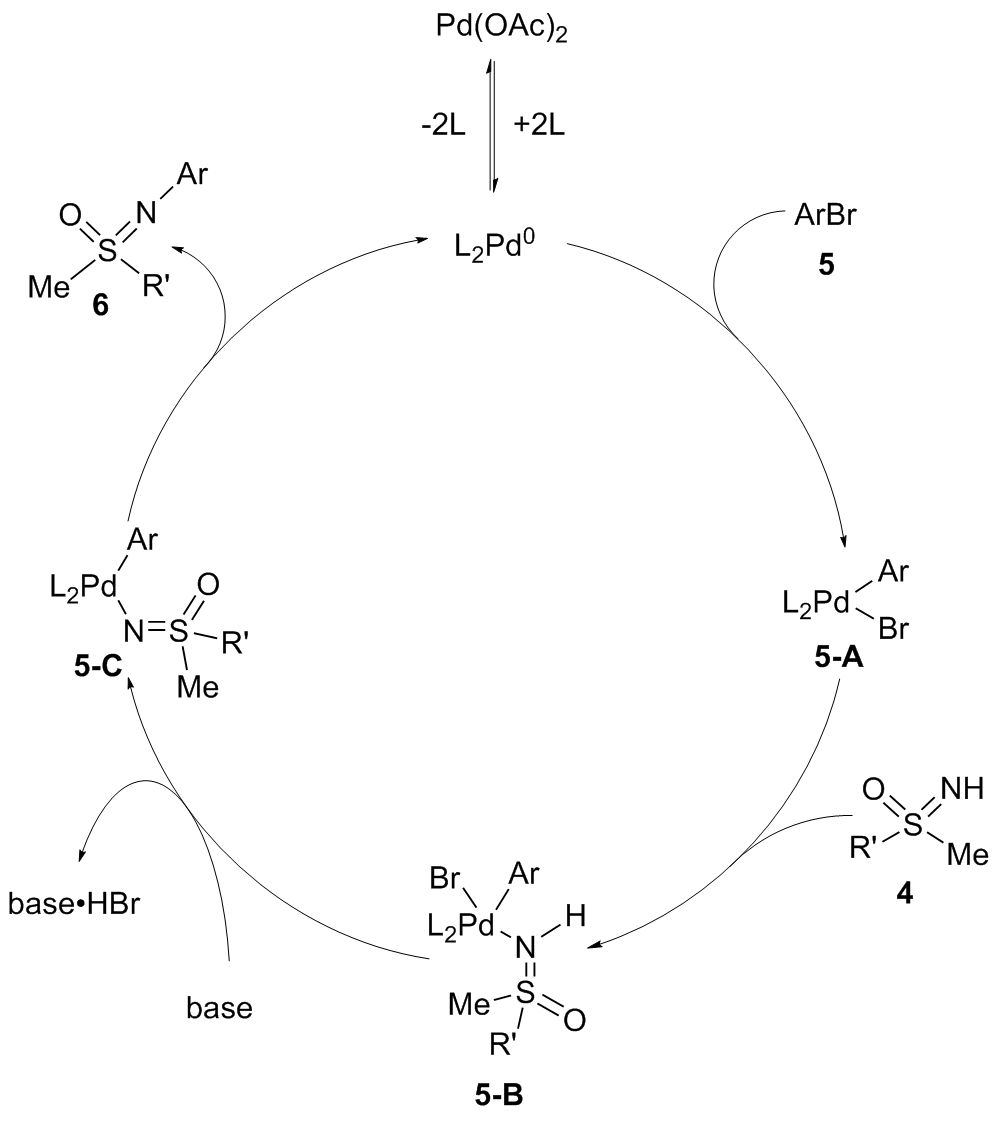
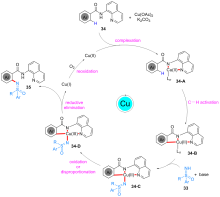
砜亚胺是有机合成中一类重要的结构单元, 可用作手性助剂、手性配体及有机催化剂等, 也是构建杂环化合物的关键中间体. 因其具有独特的生物活性, 在医药与农药方面也得到了广泛应用. 鉴于砜亚胺在有机化学以及药物研发中的重要作用, 其合成方法备受关注. 其中, 用NH-砜亚胺芳基化策略制备N-芳基砜亚胺的方法, 由于具有原子经济高、条件温和及反应路线短等优势, 受到了化学家越来越多的关注, 并取得了长足的进步. 系统介绍了NH-砜亚胺通过C—N键的构建来合成N-芳基砜亚胺的各类方法, 及其在有机合成方面的应用.
李雪, 王聪, 贾铁争. 砜亚胺N-芳基化的研究进展及其应用[J]. 有机化学, 2022, 42(3): 714-731.
Xue Li, Cong Wang, Tiezheng Jia. Recent Advances in N-Arylation of NH-Sulfoximines and Their Applications[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 714-731.
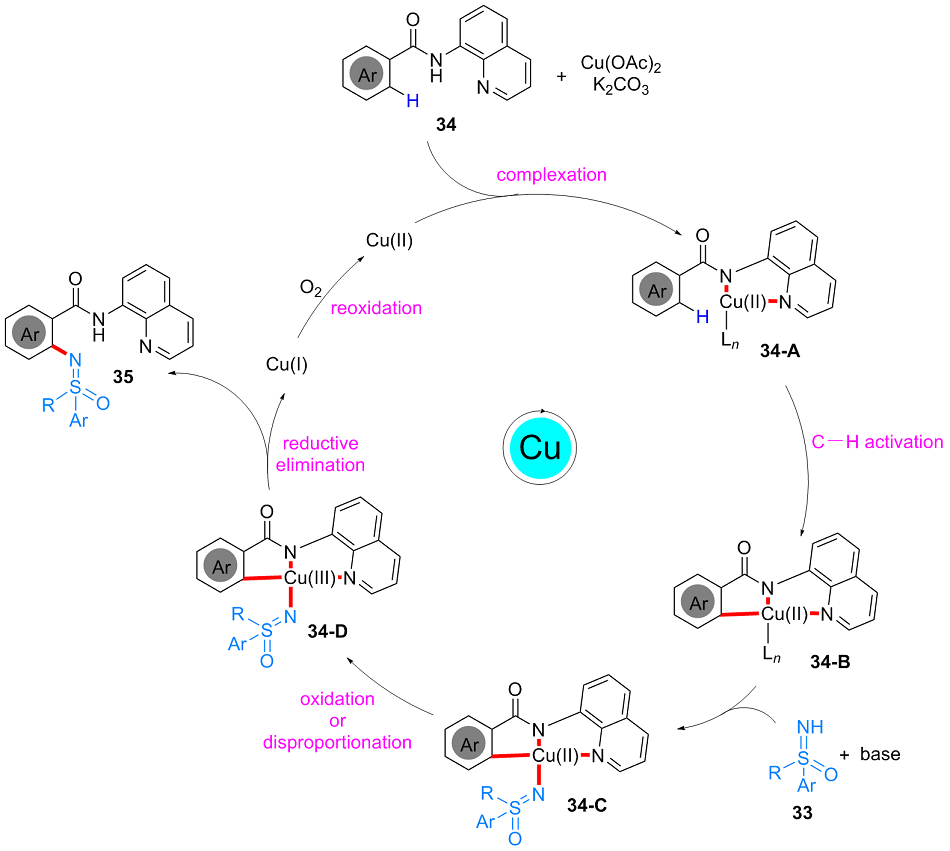

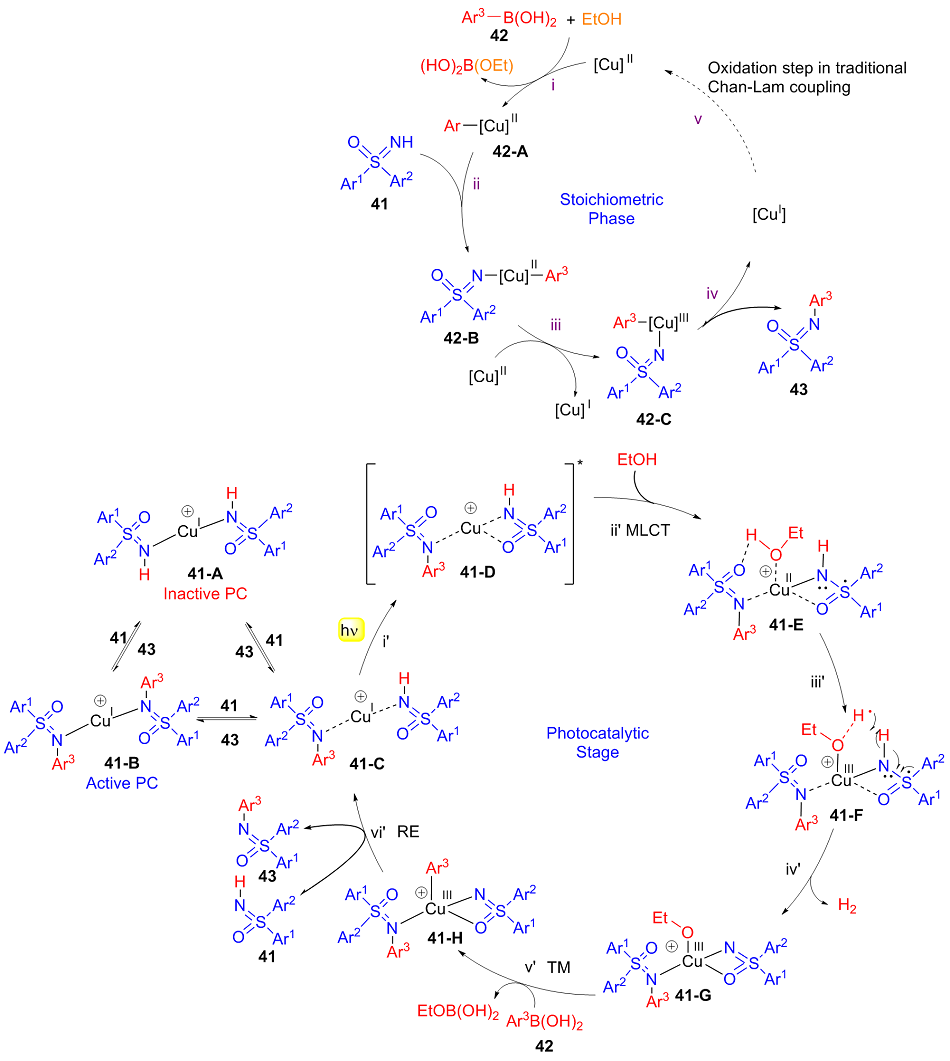
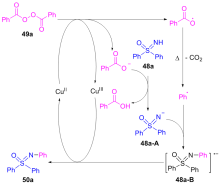
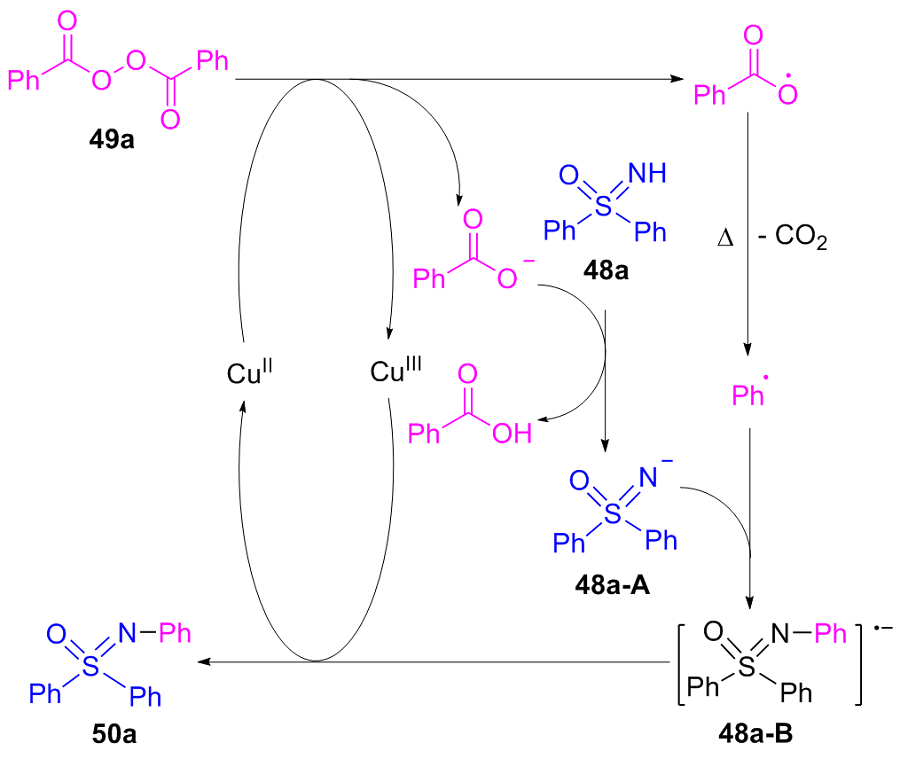
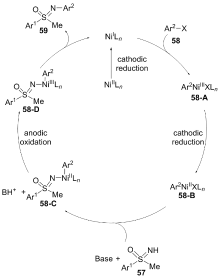


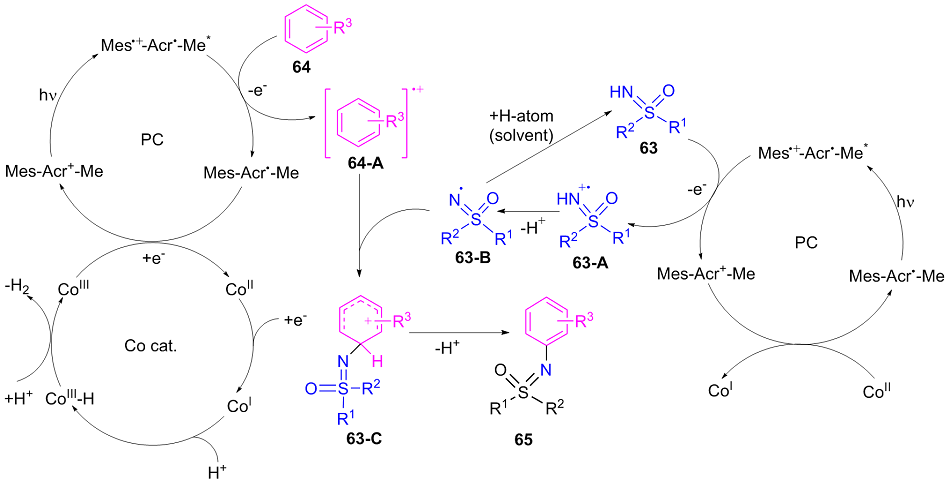

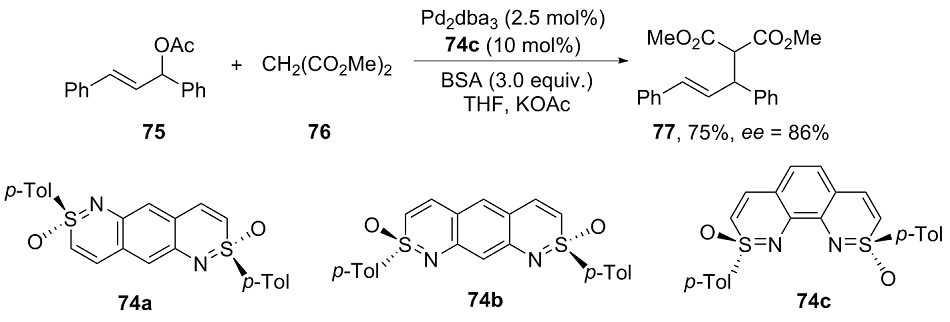

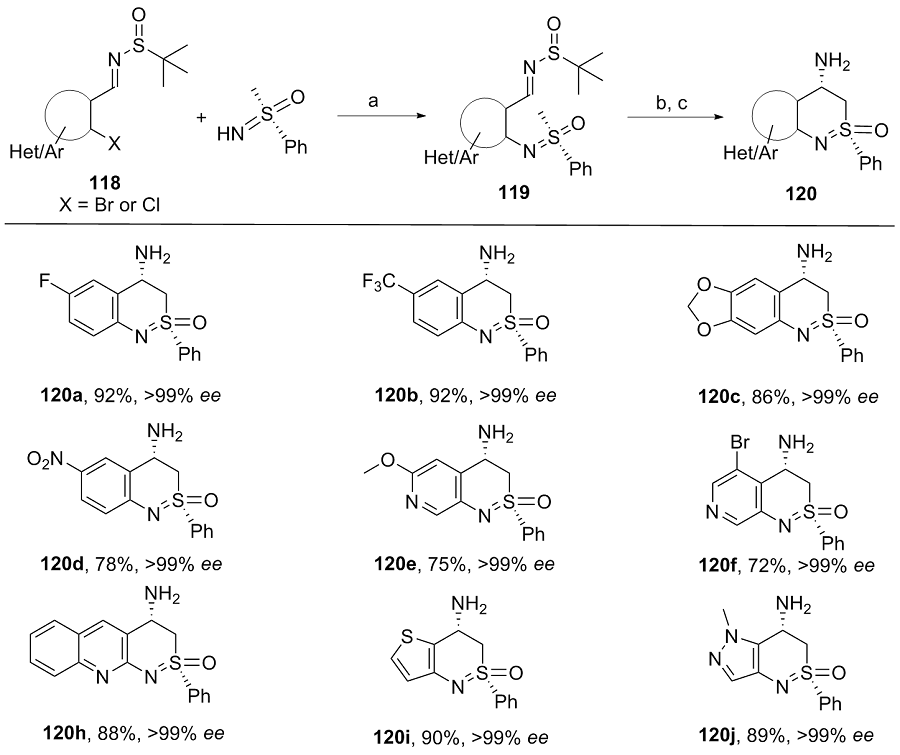
| [1] |
Lücking, U. Angew. Chem., Int. Ed. 2013, 52, 9399.
doi: 10.1002/anie.v52.36 |
| [2] |
Lücking, U.; Jautelat, R.; Krüger, M.; Brumby, T.; Lienau, P.; Schäfer, M.; Briem, H.; Schulze, J.; Hillisch, A.; Reichel, A.; Wengner, A. M.; Siemeister, G. ChemMedChem 2013, 8, 1021.
doi: 10.1002/cmdc.201390025 |
| [3] |
Zhu, Y.; Loso, M. R.; Watson, G. B.; Sparks, T. C.; Rogers, R. B.; Huang, J. X.; Gerwick, B. C.; Babcock, J. M.; Kelley, D.; Hegde, V. B.; Nugent, B. M.; Renga, J. M.; Denholm, I.; Gorman, K.; DeBoer, G. J.; Hasler, J.; Meade, T.; Thomas, J. D. J. Agric. Food Chem. 2011, 59, 2950.
doi: 10.1021/jf102765x |
| [4] |
(a) Bizet, V.; Hendriks, C. M.; Bolm, C. Chem. Soc. Rev. 2015, 44, 3378.
doi: 10.1039/C5CS00208G |
|
(b) Schafer, S.; Wirth, T. Angew. Chem., Int. Ed. 2010, 49, 2786.
doi: 10.1002/anie.200907134 |
|
|
(c) Tota, A.; Zenzola, M.; Chawner, S. J.; John-Campbell, S. S.; Carlucci, C.; Romanazzi, G.; Degennaro, L.; Bull, J. A.; Luisi, R. Chem. Commun. 2016, 53, 348.
doi: 10.1039/C6CC08891K |
|
| [5] |
Bolm, C.; Hildebrand, J. P. Tetrahedron Lett. 1998, 39, 5731.
doi: 10.1016/S0040-4039(98)01199-X |
| [6] |
Bolm, C.; Hildebrand, J. P. J. Org. Chem. 2000, 65, 169.
pmid: 10813912 |
| [7] |
Harmata, M.; Hong, X. Synlett 2007, 6, 969.
|
| [8] |
Yongpruksa, N.; Calkins, N. L.; Harmata, M. Chem. Commun. 2011, 47, 7665.
doi: 10.1039/c1cc12444g |
| [9] |
Yang, Q.; Choy, P. Y.; Zhao, Q.; Leung, M. P.; Chan, H. S.; So, C. M.; Wong, W. T.; Kwong, F. Y. J. Org. Chem. 2018, 83, 11369.
doi: 10.1021/acs.joc.8b01599 |
| [10] |
Cho, G. Y.; Remy, P.; Jansson, J.; Moessner, C.; Bolm, C. Org. Lett. 2004, 6, 3293.
doi: 10.1021/ol048806h |
| [11] |
Sedelmeier, J.; Bolm, C. J. Org. Chem. 2005, 70, 6904.
pmid: 16095312 |
| [12] |
Vaddula, B.; Leazer, J.; Varma, R. S. Adv. Synth. Catal. 2012, 354, 986.
doi: 10.1002/adsc.201100808 |
| [13] |
Miyasaka, M.; Hirano, K.; Satoh, T.; Kowalczyk, R.; Bolm, C.; Miura, M. Org. Lett. 2011, 13, 359.
doi: 10.1021/ol102844q pmid: 21174416 |
| [14] |
Wang, L.; Priebbenow, D. L.; Dong, W.; Bolm, C. Org. Lett. 2014, 16, 2661.
doi: 10.1021/ol500963p |
| [15] |
Grandhi, G. S.; Dana, S.; Mandal, A.; Baidya, M. Org. Lett. 2020, 22, 2606.
doi: 10.1021/acs.orglett.0c00545 pmid: 32180411 |
| [16] |
Moessner, C.; Bolm, C. Org. Lett. 2005, 7, 2667.
doi: 10.1021/ol050816a |
| [17] |
Gupta, S.; Baranwal, S.; Muniyappan, N.; Sabiah, S.; Kandasamy, J. Synthesis 2019, 51, 2171.
doi: 10.1055/s-0037-1612216 |
| [18] |
Wang, C.; Zhang, H.; Wells, L. A.; Liu, T.; Meng, T.; Liu, Q.; Walsh, P. J.; Kozlowski, M. C.; Jia, T. Nat. Commun. 2021, 12, 932.
doi: 10.1038/s41467-021-21156-w |
| [19] |
Kim, J.; Ok, J.; Kim, S.; Choi, W.; Lee, P. H. Org. Lett. 2014, 16, 4602.
doi: 10.1021/ol502174n |
| [20] |
Zhu, H.; Teng, F.; Pan, C.; Cheng, J.; Yu, J.-T. Tetrahedron Lett. 2016, 57, 2372.
doi: 10.1016/j.tetlet.2016.04.042 |
| [21] |
Hande, S.; Mfuh, A.; Throner, S.; Wu, Y.; Ye, Q.; Zheng, X. Tetrahedron Lett. 2019, 60, 151100.
doi: 10.1016/j.tetlet.2019.151100 |
| [22] |
Wimmer, A.; König, B. Org. Lett. 2019, 21, 2740.
doi: 10.1021/acs.orglett.9b00698 |
| [23] |
Liu, D.; Liu, Z. R.; Ma, C.; Jiao, K. J.; Sun, B.; Wei, L.; Lefranc, J.; Herbert, S.; Mei, T. S. Angew. Chem., Int. Ed. 2021, 60, 9444.
doi: 10.1002/anie.v60.17 |
| [24] |
Correa, A.; Bolm, C. Adv. Synth. Catal. 2008, 350, 391.
doi: 10.1002/(ISSN)1615-4169 |
| [25] |
Wimmer, A.; König, B. Adv. Synth. Catal. 2018, 360, 3277.
doi: 10.1002/adsc.v360.17 |
| [26] |
Aithagani, S. K.; Dara, S.; Munagala, G.; Aruri, H.; Yadav, M.; Sharma, S.; Vishwakarma, R. A.; Singh, P. P. Org. Lett. 2015, 17, 5547.
doi: 10.1021/acs.orglett.5b02804 pmid: 26562479 |
| [27] |
Meier, R.; Hog, D.; Lämmermann, H.; Sudau, A.; Rackl, D.; Weinmann, H.; Collins, K.; Wortmann, L.; Candish, L. Synlett 2018, 29, 2679.
doi: 10.1055/s-0037-1609656 |
| [28] |
Harmata, M.; Pavri, N. Angew. Chem., Int. Ed. 1999, 38, 2577.
doi: 10.1002/(ISSN)1521-3773 |
| [29] |
Harmata, M.; Ghosh, S. K. Org. Lett. 2001, 3, 3321.
pmid: 11594824 |
| [30] |
Bolm, C.; Simic, O. J. Am. Chem. Soc. 2001, 123, 3830.
pmid: 11457120 |
| [31] |
Bolm, C.; Martin, M.; Simic, O.; Verrucci, M. Org. Lett. 2003, 5, 427.
doi: 10.1021/ol027273e |
| [32] |
Langner, M.; Bolm, C. Angew. Chem., Int. Ed. 2004, 43, 5984.
doi: 10.1002/(ISSN)1521-3773 |
| [33] |
Langner, M.; Remy, P.; Bolm, C. Chem.-Eur. J. 2005, 11, 6254.
pmid: 16075444 |
| [34] |
Frings, M.; Atodiresei, I.; Wang, Y.; Runsink, J.; Raabe, G.; Bolm, C. Chem.-Eur. J. 2010, 16, 4577.
doi: 10.1002/chem.v16:15 |
| [35] |
Moessner, C.; Bolm, C. Angew. Chem., Int. Ed. 2005, 44, 7564.
doi: 10.1002/(ISSN)1521-3773 |
| [36] |
Biosca, M.; Pἁmies, O.; Diéguez, M. J. Org. Chem. 2019, 84, 8259.
doi: 10.1021/acs.joc.9b00829 |
| [37] |
Harmata, M.; Hong, X. J. Am. Chem. Soc. 2003, 125, 5754.
doi: 10.1021/ja034744z |
| [38] |
Harmata, M.; Hong, X. Org. Lett. 2007, 9, 2701.
doi: 10.1021/ol0710358 |
| [39] |
Battula, S. R. K.; Subbareddy, G. V.; Chakravarthy, I. E.; Saravanan, V. RSC Adv. 2016, 6, 55710.
doi: 10.1039/C6RA08590C |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||