有机化学 ›› 2023, Vol. 43 ›› Issue (7): 2407-2424.DOI: 10.6023/cjoc202210035 上一篇 下一篇
综述与进展
收稿日期:2022-10-28
修回日期:2023-01-16
发布日期:2023-02-06
通讯作者:
周林
基金资助:
Lin Zhou( ), Hong Yang, Chuan Yang, Zhigang Zhao, Qinghan Li
), Hong Yang, Chuan Yang, Zhigang Zhao, Qinghan Li
Received:2022-10-28
Revised:2023-01-16
Published:2023-02-06
Contact:
Lin Zhou
Supported by:文章分享
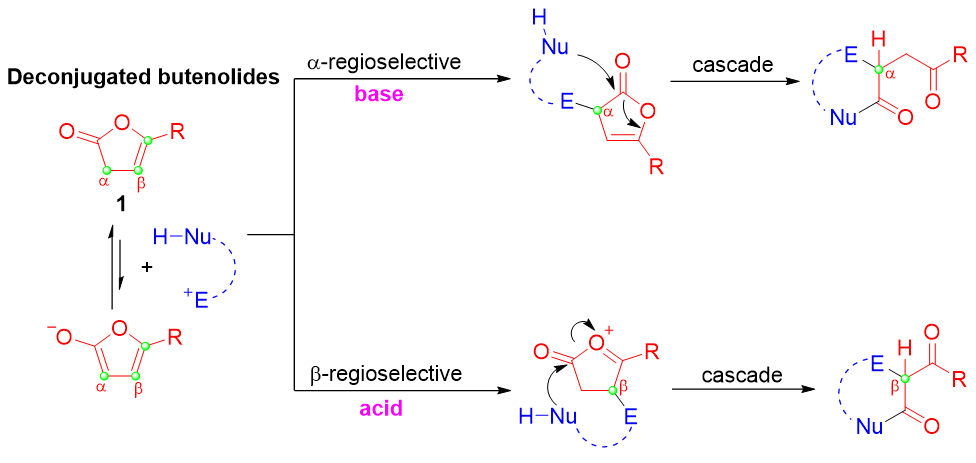
γ-内酯衍生物大多具有抗炎、抗肿瘤和抗癌等生物活性, 发展γ-内酯衍生物的构建方法在有机合成中具有重要价值. 其中, 以非共轭丁烯内酯为起始原料的构建方案呈现出巨大的潜力和无可比拟的优势, 是最直接、高效的方法, 受到化学家的广泛关注, 是近年来有机合成研究热点之一. 综述了非共轭丁烯内酯α-和β-位参与的反应研究进展, 重点概述了各类反应体系的特点, 并对部分反应的机理进行了讨论. 同时, 对该领域未来发展方向进行了展望.
周林, 杨鸿, 杨川, 赵志刚, 李清寒. 非共轭丁烯内酯α-和β-位反应研究进展[J]. 有机化学, 2023, 43(7): 2407-2424.
Lin Zhou, Hong Yang, Chuan Yang, Zhigang Zhao, Qinghan Li. Advances on the α- and β-Reactions of Deconjugated Butenolides[J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2407-2424.
| [1] |
For selected reviews and examples, see: (a) Kitson, R.R.A.; Millemaggi, A.; Taylor, R., J. K. Angew. Chem., Int. Ed. 2009, 48, 9426.
pmid: 16468720 |
|
(b) Lone, S. H.; Bhat, K. A.; Khuroo, M. A. Chem.-Biol. Interact. 2015, 240, 180.
doi: 10.1016/j.cbi.2015.08.015 pmid: 16468720 |
|
|
(c) Yu, X.; Che, Z.; Xu, H. Chem.-Eur. J. 2017, 23, 4467.
doi: 10.1002/chem.201602472 pmid: 16468720 |
|
|
(d) Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. Chem. Rev. 2020, 120, 2448.
doi: 10.1021/acs.chemrev.9b00481 pmid: 16468720 |
|
|
(e) Choudhury, A. R.; Mukherjee, S. Chem. Soc. Rev. 2020, 49, 6755.
doi: 10.1039/c9cs00346k pmid: 16468720 |
|
|
(f) Boeckman, R. K.; Pero, J. E.; Boehmler, D. J. J. Am. Chem. Soc. 2006, 128, 11032.
pmid: 16468720 |
|
|
(g) Robichaud, J.; Tremblay, F. Org. Lett. 2006, 8, 597.
pmid: 16468720 |
|
|
(h) Zhang, X.; Yin, Y.; Zhou, Y.; Zhu, T.; Wang, M.; Gao, H. Chin. J. Chem. 2022, 40, 617.
doi: 10.1002/cjoc.v40.5 pmid: 16468720 |
|
|
(i) You, J.-Q.; Liu, Y.-N.; Zhou, J.-S.; Sun, X.-Y.; Lei, C.; Mu, Q.; Li, J.-Y.; Hou, A.-J. Chin. J. Chem. 2022, 40, 2882.
doi: 10.1002/cjoc.v40.24 pmid: 16468720 |
|
|
(j) Zhang, H.; Nan, F. Chin. J. Chem. 2013, 31, 84.
doi: 10.1002/cjoc.201200695 pmid: 16468720 |
|
|
(k) Tang, B.; Guan, A.; Zhao, Y.; Jiang, J.; Wang, M.; Zhou, L. Chin. J. Chem. 2017, 35, 1133.
doi: 10.1002/cjoc.v35.7 pmid: 16468720 |
|
|
(l) Wang, Y.; Chen, B.; He, X.; Gui, J. Chin. J. Chem. 2020, 38, 1339.
doi: 10.1002/cjoc.v38.11 pmid: 16468720 |
|
| [2] |
(a) Ma, S.-M.; Shi, Z.-J. Chin. J. Chem. 2001, 19, 1280.
doi: 10.1002/cjoc.20010191220 |
|
(b) Wang, J. P.; Chang, X. H.; Tian, X. Z.; Chen, Q. H. Acta Chim. Sinica 2003, 61, 411 (in Chinese).
|
|
|
(王建平, 常新红, 田欣哲, 陈庆华, 化学学报, 2003, 61, 411.)
|
|
|
(c) Zhao, Y.; Jiang, S.; Guo, Y.-W.; Yao, Z.-J. Chin. J. Chem. 2005, 23, 173.
doi: 10.1002/(ISSN)1614-7065 |
|
|
(d) Zhao, Y.; Tang, B.; Liu, X.; Li, W. Z.; Huang, M. Y.; Wang, M. A. Chin. J. Org. Chem. 2017, 37, 975 (in Chinese).
doi: 10.6023/cjoc201611013 |
|
|
(赵宇, 汤博, 刘鑫磊, 李婉祯, 黄铭一, 王明安, 有机化学, 2017, 37, 975.)
doi: 10.6023/cjoc201611013 |
|
|
(e) Li, X.; Zhu, K.; Han, Q.; Lu, X. X.; Li, M. J.; Ling, Y.; Duan, H. X. Chin. J. Org. Chem. 2022, 42, 202 (in Chinese).
|
|
|
(李想, 朱凯, 韩清, 路星星, 李明君, 凌云, 段红霞, 有机化学, 2022, 42, 202.)
|
|
|
(f) Zhang, Q.; Li, Y. H.; Xu, L. C.; Ma, H. Y.; Li, X. D.; Wang, M. A. Chin. J. Org. Chem. 2022, 42, 2438 (in Chinese).
doi: 10.6023/cjoc202203033 |
|
|
(张倩, 李益豪, 许磊川, 马好运, 李向东, 王明安, 有机化学, 2022, 42, 2438.)
doi: 10.6023/cjoc202203033 |
|
| [3] |
(a) Das, U.; Chen, Y.-R.; Tsai, Y.-L.; Lin, W. Chem.-Eur. J. 2013, 19, 7713.
doi: 10.1002/chem.v19.24 |
|
(b) Rout, S.; Das, A.; Singh, V. K. Chem. Commun. 2017, 53, 5143.
doi: 10.1039/C7CC01763D |
|
| [4] |
(a) Adekenov, S. M.; Mukhametzhanov, M. N.; Kagarlitskii, A. D.; Kupriyanov, A. N. Khim. Prir. Soedin. 1982, 655.
|
|
(b) Zhangabylov, N. S.; Dederer, L. Y.; Gorbacheva, L. B.; Vasilêva, S. V.; Terekhov, A. S.; Adekenov, S. M. Pharm. Chem. J. 2004, 38, 651.
doi: 10.1007/s11094-005-0052-9 |
|
| [5] |
Aubert, S.; Katsina, T.; Arseniyadis, S. Org. Lett. 2019, 21, 2231.
doi: 10.1021/acs.orglett.9b00521 |
| [6] |
For selected reviews, see: (a) Jusseau, X.; Chabaud, L.; Guillou, C. Tetrahedron 2014, 70, 2595.
doi: 10.1016/j.tet.2014.01.057 |
|
(b) Roselló, M. S.; del Pozoa, C.; Fustero, S. Synthesis 2016, 48, 2553.
doi: 10.1055/s-0035-1561650 |
|
|
(c) Mao, B.; Fañanas-Mastral, M.; Feringa, B. L. Chem. Rev. 2017, 117, 10502.
doi: 10.1021/acs.chemrev.7b00151 |
|
|
(d) Yin, Y.; Jiang, Z. ChemCatChem 2017, 9, 4306.
doi: 10.1002/cctc.v9.23 |
|
|
(e) Li, H.; Yin, L. Tetrahedron Lett. 2018, 59, 4121.
doi: 10.1016/j.tetlet.2018.10.012 |
|
| [7] |
For selected examples on activated silyloxyfurans, see: (a) Redero, E.; Sandoval, C.; Bermejo, F. Tetrahedron 2001, 57, 9597.
pmid: 20583835 |
|
(b) Singh, R. P.; Foxman, B. M.; Deng, L. J. Am. Chem. Soc. 2010, 132, 9558.
doi: 10.1021/ja103331t pmid: 20583835 |
|
|
(c) Meshram, H. M.; Ramesh, P.; Reddy, B. C.; Sridhar, B.; Yadav, J. S. Tetrahedron 2011, 67, 3150.
doi: 10.1016/j.tet.2011.02.033 pmid: 20583835 |
|
|
(d) Mao, B.; Ji, Y.; Fananas-Mastral, M.; Caroli, G.; Meetsma, A.; Feringa, B. L. Angew. Chem., Int. Ed. 2012, 51, 3168.
doi: 10.1002/anie.v51.13 pmid: 20583835 |
|
|
(e) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 15249.
doi: 10.1021/ja306850b pmid: 20583835 |
|
|
(f) Woyciechowska, M.; Forcher, G.; Buda, S.; Mlynarski, J. Chem. Commun. 2012, 48, 11029.
doi: 10.1039/c2cc36656h pmid: 20583835 |
|
|
(g) Kong, S.; Fan, W.; Lyu, H.; Zhan, J.; Miao, X.; Miao, Z. Synth. Commun. 2014, 44, 936.
doi: 10.1080/00397911.2013.837926 pmid: 20583835 |
|
|
(h) Wang, Y.; Xing, F.; Xue, M.; Du, G.-F.; Guo, X.-H.; Huang, K.-W.; Dai, B. Synthesis 2016, 48, 79.
doi: 10.1055/s-00000084 pmid: 20583835 |
|
|
(i) Adamkiewicz, A.; Węglarz, I.; Butkiewicz, A.; Woyciechowska, M.; Mlynarski, J. Adv. Synth. Catal. 2020, 362, 667.
doi: 10.1002/adsc.v362.3 pmid: 20583835 |
|
| [8] |
(a) Zhang, Y.; Yu, C.; Ji, Y.; Wang, W. Chem.-Asian J. 2010, 5, 1303.
|
|
(b) Huang, H.; Yu, F.; Jin, Z.; Li, W.; Wu, W.; Liang, X.; Ye, J. Chem. Commun. 2010, 46, 5957.
doi: 10.1039/c0cc01054e |
|
|
(c) Luo, J.; Wang, H.; Han, X.; Xu, L.-W.; Kwiatkowski, J.; Huang, K.-W.; Lu, Y. Angew. Chem., Int. Ed. 2011, 50, 1861.
doi: 10.1002/anie.v50.8 |
|
|
(d) Yin, L.; Takada, H.; Lin, S.; Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2014, 53, 5327.
doi: 10.1002/anie.v53.21 |
|
| [9] |
(a) Cui, H. L.; Huang, J. R.; Lei, J.; Wang, Z. F.; Chen, S.; Wu, L.; Chen, Y. C. Org. Lett. 2010, 12, 720.
doi: 10.1021/ol100014m |
|
(b) Wu, Y.; Singh, R. P.; Deng, L. J. Am. Chem. Soc. 2011, 133, 12458.
doi: 10.1021/ja205674x |
|
|
(c) Griswold, J. A.; Johnson, J. S. ACS Catal. 2019, 9, 11614.
doi: 10.1021/acscatal.9b04405 |
|
|
(d) Yu, C.; Ji, P.; Zhang, Y.; Meng, X.; Wang, W. Org. Lett. 2021, 23, 7656.
doi: 10.1021/acs.orglett.1c02916 |
|
|
(e) Dai, Z.-Y.; Wang, P.-S.; Gong, L.-Z. Chem. Commun. 2021, 57, 6748.
doi: 10.1039/D1CC02295D |
|
| [10] |
(a) Helberger, V. J. H.; Ulubey, S.; Civelekoglu, H. Justus Liebigs Ann. Chem. 1949, 561, 215.
doi: 10.1002/(ISSN)1099-0690 |
|
(b) Karwa, S.; Gajiwala, V. M.; Heltzel, J.; Patil, S. K. R.; Lund, C. R. F. Catal. Today 2016, 263, 16.
doi: 10.1016/j.cattod.2015.06.020 |
|
| [11] |
Pelter, A.; Rowlands, M. Tetrahedron Lett. 1987, 28, 1203.
|
| [12] |
Marshall, J. A.; Wolf, M. A.; Wallace, E. M. J. Org. Chem. 1997, 62, 367.
pmid: 11671411 |
| [13] |
(a) Osman, N. A.; Mahmoud, A. H.; Allara, M.; Niess, R.; Abouzid, K. A.; Marzo, V. D.; Abadi, A. H. Bioorg. Med. Chem. 2010, 18, 8463.
doi: 10.1016/j.bmc.2010.10.050 pmid: 19606900 |
|
(b) Muratore, M. E.; Holloway, C. A.; Pilling, A. W.; Storer, R. I.; Trevitt, G.; Dixon, D. J. J. Am. Chem. Soc. 2009, 131, 10796.
doi: 10.1021/ja9024885 pmid: 19606900 |
|
| [14] |
Lima, C. G. S.; Monteiro, J. L.; Lima, T. Paixão, M. W.; Corrêa, A. G. ChemSusChem. 2018, 11, 25.
doi: 10.1002/cssc.v11.1 |
| [15] |
For selected recent reports on the γ-addition of deconjugated butenolides, see: (a) Quintard, A.; Lefranc, A.; Alexakis, A. Org. Lett. 2011, 13, 1540.
doi: 10.1021/ol200235j pmid: 22952199 |
|
(b) Zhou, L.; Lin, L.-L.; Ji, J.; Xie, M.-S.; Liu, X.-H.; Feng, X.-M. Org. Lett. 2011, 13, 3056.
doi: 10.1021/ol200939t pmid: 22952199 |
|
|
(c) Zhang, W.; Tan, D.; Lee, R.; Tong, G.; Chen, W.; Qi, B.; Huang, K.-W.; Tan, C.-H.; Jiang, Z. Angew. Chem., Int. Ed. 2012, 51, 10069.
doi: 10.1002/anie.201205872 pmid: 22952199 |
|
|
(d) Manna, M. S.; Kumar, V.; Mukherjee, S. Chem. Commun. 2012, 48, 5193.
doi: 10.1039/C2CC31700A pmid: 22952199 |
|
|
(e) Manna, M. S.; Mukherjee, S. Chem.-Eur. J. 2012, 18, 15277.
doi: 10.1002/chem.v18.48 pmid: 22952199 |
|
|
(f) Ji, J.; Lin, L.-L.; Zhou, L.; Zhang, Y.-H.; Liu, Y.-B.; Liu, X.-H.; Feng X.-M. Adv. Synth. Catal. 2013, 355, 2764.
pmid: 22952199 |
|
|
(g) Yang, D.; Wang, L.; Zhao, D.; Han, F.; Zhang, B.; Wang, R. Chem.-Eur. J. 2013, 19, 4691.
doi: 10.1002/chem.201204466 pmid: 22952199 |
|
| [16] |
For selected reports on the γ-addition of deconjugated butenolides, see: (a) Guo, Y.-L.; Jia, L.-N.; Peng, L.; Qi, L.-W.; Zhou, J.; Tian, F.; Xu, X.-Y.; Wang, L. -X. RSC Adv. 2013, 3, 16973.
doi: 10.1039/c3ra43344g |
|
(b) Kumar, V.; Ray, B.; Rathi, P.; Mukherjee, S. Synthesis 2013, 45, 1641.
doi: 10.1055/s-00000084 |
|
|
(c) Manna, M. S.; Mukherjee, S. Chem. Sci. 2014, 5, 1627.
doi: 10.1039/C3SC53102C |
|
|
(d) Li, X.; Lu, M.; Dong, Y.; Wu, W.; Qian, Q.; Ye, J.; Dixon, D. J. Nat. Commun. 2014, 4, 4479.
|
|
|
(e) Wang, Z.-H.; Wu, Z.-J.; Huang, X.-Q.; Yue, D.-F.; You, Y.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Chem. Commun. 2015, 51, 15835.
doi: 10.1039/C5CC06383C |
|
|
(f) Guo, H.; Xing, F.; Du, G.-F.; Huang, K.-W.; Dai, B.; He, L. J. Org. Chem. 2015, 80, 12606.
doi: 10.1021/acs.joc.5b01845 |
|
| [17] |
For selected reports on the γ-addition of deconjugated butenolides, see: (a) Sekikawa, T.; Kitaguchi, T.; Kitaura, H.; Minami, T.; Hatanaka, Y. Org. Lett. 2015, 17, 3026.
doi: 10.1021/acs.orglett.5b01224 pmid: 26646367 |
|
(b) Lagoutte, R.; Besnard, C.; Alexakis, A. Eur. J. Org. Chem. 2016, 4372.
pmid: 26646367 |
|
|
(c) Simlandy, A. K.; Mukherjee, S. Org. Biomol. Chem. 2016, 14, 5659.
doi: 10.1039/c5ob02362a pmid: 26646367 |
|
|
(d) Tang, Q.; Lin, L.-L.; Ji, J.; Hu, H.-P.; Liu, X.-H.; Feng, X.-M. Chem.-Eur. J. 2017, 23, 16447.
doi: 10.1002/chem.v23.65 pmid: 26646367 |
|
|
(e) Ji, J.; Lin, L.-L.; Tang, Q.; Kang, T.-F.; Liu, X.-H.; Feng, X.-M. ACS Catal. 2017, 7, 3763.
doi: 10.1021/acscatal.7b00590 pmid: 26646367 |
|
|
(f) Trost, B. M.; Gnanamani, E.; Tracy, J. S.; Kalnmals, C. J. Am. Chem. Soc. 2017, 139, 18198.
doi: 10.1021/jacs.7b11361 pmid: 26646367 |
|
|
(g) Rout, S.; Joshi, H.; Singh, V. K. Org. Lett. 2018, 20, 2199.
doi: 10.1021/acs.orglett.8b00493 pmid: 26646367 |
|
| [18] |
For selected reports on the γ-addition of deconjugated butenolides, see: (a) Sakai, T.; Hirashima, S.; Matsushima, Y.; Nakano, T.; Ishii, D.; Yamashita, Y.; Nakashima, K.; Koseki, Y.; Miura, T. Org. Lett. 2019, 21, 2606.
doi: 10.1021/acs.orglett.9b00574 |
| [19] |
Jefford, C. W.; Jaggi, D.; Boukouvalas, J. J. Chem. Soc., Chem. Commun. 1988, 1595.
|
| [20] |
Egorova, A. Y.; Timofeeva, Z. Y. Russ. J. Gen. Chem. 2003, 73, 655.
doi: 10.1023/A:1025625511411 |
| [21] |
Griswold, J. A.; Horwitz, M. A.; Leiva, L. V.; Johnson, J. S. J. Org. Chem. 2017, 82, 2276.
doi: 10.1021/acs.joc.6b03059 pmid: 28164699 |
| [22] |
(a) Mengel, A.; Reiser, O. Chem. Rev. 1999, 99, 1191.
pmid: 11749444 |
|
(b) Evans, D. A.; Siska, S. J.; Cee, V. J. Angew. Chem., Int. Ed. 2003, 42, 1761.
doi: 10.1002/anie.200350979 pmid: 11749444 |
|
|
(c) Cee, V. J.; Cramer, C. J.; Evans, D. A. J. Am. Chem. Soc. 2006, 128, 2920.
doi: 10.1021/ja0555670 pmid: 11749444 |
|
| [23] |
Yang, M.; Chen, C.; Yi, X.; Li, Y.; Wu, X.; Li, Q.; Ban, S. Org. Biomol. Chem. 2019, 17, 2883.
doi: 10.1039/C9OB00330D |
| [24] |
Li, Feng.; Wang, J.; Pei, W.; Ma, H.; Li, H.; Cui, M.; Peng, S.; Wang, S.; Liu, L. Tetrahedron Lett. 2018, 59, 3010.
doi: 10.1016/j.tetlet.2018.06.062 |
| [25] |
Trost, B. M.; Joey Hung, C.-I.; Scharf, M. J. Angew. Chem., Int. Ed. 2018, 57, 11408.
doi: 10.1002/anie.v57.35 |
| [26] |
Huang, Z-Y.; Yang, H.; Zhou, L.; Li, Q.-H.; Zhao, Z.-G. Tetrahedron. 2022, 112, 132740.
doi: 10.1016/j.tet.2022.132740 |
| [27] |
Wu, Bo.; Yu, Z.; Gao, X.; Lan, Y.; Zhou, Y.-G. Angew. Chem., Int. Ed. 2017, 56, 4006.
doi: 10.1002/anie.201700437 pmid: 28247568 |
| [28] |
(a) Green, A. G.; Liu, P.; Merlic, C. A.; Houk, K. N. J. Am. Chem. Soc. 2014, 136, 4575.
doi: 10.1021/ja411699u |
|
(b) Wang, T.; Yu, Z.; Hoon, D. L.; Phee, C. Y.; Lan, Y.; Lu, Y. J. Am. Chem. Soc. 2016, 138, 265.
doi: 10.1021/jacs.5b10524 |
|
| [29] |
Wang, Y.-N.; Xiong, Q.; Lu, L.-Q.; Zhang, Q.-L.; Wang, Y.; Lan, Y.; Xiao, W.-J. Angew. Chem., Int. Ed. 2019, 58, 11013.
doi: 10.1002/anie.v58.32 |
| [30] |
Xu, Y.-W.; Hu, X.-P. Org. Lett. 2019, 21, 8091.
doi: 10.1021/acs.orglett.9b03081 |
| [31] |
Hattori, G.; Sakata, K.; Matsuzawa, H.; Tanabe, Y.; Miyake, Y.; Nishibayashi, Y. J. Am. Chem. Soc. 2010, 132, 10592.
doi: 10.1021/ja1047494 pmid: 20617844 |
| [32] |
Zhao, J.-Q.; Luo, S.-W.; Zhang, X.-M.; Xu, X.-Y.; Zhou, M.-Q.; Yuan, W.-C. Tetrahedron 2017, 73, 5444.
doi: 10.1016/j.tet.2017.07.053 |
| [33] |
Sharma, B. M.; Shinde, D. R.; Jain, R.; Begari, E.; Satbhaiya, S.; Gonnade, R. G.; Kumar, P. Org. Lett. 2018, 20, 2787.
doi: 10.1021/acs.orglett.8b00745 |
| [34] |
Masuyama, Y.; Kobayashi, Y.; Yanagi, R.; Kurusu, Y. Chem. Lett. 1992, 2039.
|
| [35] |
Isambert, N.; Cruz, M.; Arévalo, M. J.; Gómez, E.; Lavilla, R. Org. Lett. 2007, 9, 4199.
pmid: 17867693 |
| [36] |
Huo, C.; Yuan, Yong. J. Org. Chem. 2015, 80, 12704.
doi: 10.1021/acs.joc.5b02354 |
| [37] |
Huo, C.; Yuan, Y.; Chen, F.; Tang, J.; Wang, Y. Org. Lett. 2015, 17, 4208.
doi: 10.1021/acs.orglett.5b01985 |
| [38] |
Zhou, H.; Yang, X.; Li, S.; Zhu, Y.; Li, Y.; Zhang, Y. Org. Biomol. Chem. 2018, 16, 6728.
doi: 10.1039/C8OB01844H |
| [39] |
(a) For reviews on nitrosocarbonyl chemistry, see: Palmer, L. I.; Frazier, C. P.; Read De Alaniz, J. Synthesis 2014, 46, 269.
doi: 10.1055/s-00000084 |
|
(b) Maji, B.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2015, 88, 753.
doi: 10.1246/bcsj.20150040 |
|
|
(c) Memeo, M. G.; Quadrelli, P. Chem. Rev. 2017, 117, 2108.
doi: 10.1021/acs.chemrev.6b00684 |
|
|
(d) Dana, S.; Ramakrishna, I.; Baidya, M. Synthesis 2017, 49, 3281.
doi: 10.1055/s-0036-1590793 |
|
| [40] |
Mallik, S.; Bhajammanavar, V.; Baidya, M. Org. Lett. 2020, 22, 1437.
doi: 10.1021/acs.orglett.0c00039 |
| [41] |
(a) Payette, J. N.; Yamamoto, H. J. Am. Chem. Soc. 2008, 130, 12276.
doi: 10.1021/ja804325f pmid: 18722431 |
|
(b) Maji, B.; Yamamoto, H. Angew. Chem., Int. Ed. 2014, 53, 14472.
doi: 10.1002/anie.201408893 pmid: 18722431 |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||