有机化学 ›› 2024, Vol. 44 ›› Issue (6): 1957-1966.DOI: 10.6023/cjoc202311013 上一篇 下一篇
研究论文
孙超a,b, 周泉b,*( ), 李传莹a,*(
), 李传莹a,*( ), 王磊b,c,d,*(
), 王磊b,c,d,*( )
)
收稿日期:2023-11-14
修回日期:2024-01-29
发布日期:2024-02-27
基金资助:
Chao Suna,b, Quan Zhoub,*( ), Chuanying Lia,*(
), Chuanying Lia,*( ), Lei Wangb,c,d,*(
), Lei Wangb,c,d,*( )
)
Received:2023-11-14
Revised:2024-01-29
Published:2024-02-27
Contact:
* Supported by:文章分享

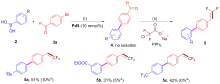
合成了6个具有柔性直链烷基或苄基连接体的七元苯并噁唑-氮杂环卡宾钯的新配合物, 用NMR, HRMS, FTIR等对其构进行了表征, 并用X射线单晶衍射进一步确认了其中3个配合物的结构. 晶体解析显示钯配位中心是稍微扭曲的平面四边形构型, 其配体中苯并噁唑和氮杂环卡宾平面互成一定角度, 不共平面. 氮杂环卡宾片段相连的第一个亚甲基上的偕二氢在配合物中表现磁各向异性, 亚甲基偕二氢化学位移差(Δδg)随侧链长度增长而变化, 侧链为四个碳的直链烷基时, Δδg最大. 催化活性应用研究显示, 此类配合物对Suzuki-Miyaura反应具有优异的催化活性, 并且可应用于两步一锅法合成联苯类偕二氟烯烃化合物.
孙超, 周泉, 李传莹, 王磊. 苯并唑-氮杂环卡宾钯配合物的合成表征及应用[J]. 有机化学, 2024, 44(6): 1957-1966.
Chao Sun, Quan Zhou, Chuanying Li, Lei Wang. Syntheses and Structural Characterizations of Benzoxazolyl N-Heterocyclic Carbene-Palladium Complexes and Their Applications[J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1957-1966.
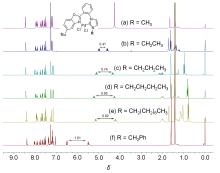

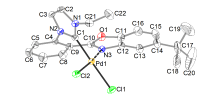

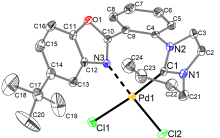

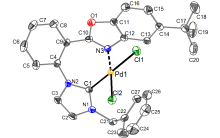

| Pd complex | Pd—H21N | Pd—H21F | Cl—H21N | Cl—H21F |
|---|---|---|---|---|
| Pd2 | 0.30878(3) | 0.43335(4) | 0.28112(10) | 0.41352(10) |
| Pd4 | 0.29863(3) | 0.42461(4) | 0.28426(13) | 0.39054(13) |
| Pd6 | 0.29519(5) | 0.42365(5) | 0.27876(12) | 0.38604(12) |
| Pd complex | Pd—H21N | Pd—H21F | Cl—H21N | Cl—H21F |
|---|---|---|---|---|
| Pd2 | 0.30878(3) | 0.43335(4) | 0.28112(10) | 0.41352(10) |
| Pd4 | 0.29863(3) | 0.42461(4) | 0.28426(13) | 0.39054(13) |
| Pd6 | 0.29519(5) | 0.42365(5) | 0.27876(12) | 0.38604(12) |
| Entry | Base | Solvent | Yield/% |
|---|---|---|---|
| 1 | K3PO4 | DMF | 87 |
| 2 | KH2PO4 | DMF | 74 |
| 3 | K2CO3 | DMF | 98 |
| 4 | Na2CO3 | DMF | 93 |
| 5 | NaHCO3 | DMF | 88 |
| 6 | N(C2H5)3 | DMF | 51 |
| 7 | K2CO3 | DMA | 95 |
| 8 | K2CO3 | DME | 30 |
| 9 | K2CO3 | MeOH | 61 |
| 10 | K2CO3 | DMF | 28b |
| Entry | Base | Solvent | Yield/% |
|---|---|---|---|
| 1 | K3PO4 | DMF | 87 |
| 2 | KH2PO4 | DMF | 74 |
| 3 | K2CO3 | DMF | 98 |
| 4 | Na2CO3 | DMF | 93 |
| 5 | NaHCO3 | DMF | 88 |
| 6 | N(C2H5)3 | DMF | 51 |
| 7 | K2CO3 | DMA | 95 |
| 8 | K2CO3 | DME | 30 |
| 9 | K2CO3 | MeOH | 61 |
| 10 | K2CO3 | DMF | 28b |
| Pd complex | Yield/% | ||
|---|---|---|---|
| 100 mmol% | 10 mmol% | 1 mmol% | |
| Pd1 | 98 | 84 | 46 |
| Pd2 | 98 | 85 | 50 |
| Pd3 | 97 | 82 | 54 |
| Pd4 | 98 | 87 | 56 |
| Pd5 | 98 | 89 | 57 |
| Pd6 | 94 | 71 | 34 |
| Pd complex | Yield/% | ||
|---|---|---|---|
| 100 mmol% | 10 mmol% | 1 mmol% | |
| Pd1 | 98 | 84 | 46 |
| Pd2 | 98 | 85 | 50 |
| Pd3 | 97 | 82 | 54 |
| Pd4 | 98 | 87 | 56 |
| Pd5 | 98 | 89 | 57 |
| Pd6 | 94 | 71 | 34 |

| [1] |
Arduengo, A. J. III; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
|
| [2] |
(a) Nolan, S. P. Angew. Chem., Int. Ed. 2015, 127, 5916.
|
|
(b) Anju, P. J.; Neetha, M.; Anilkumar, G. ChemistrySelect 2022, 7, e202103564.
|
|
|
(c) Budagumpi, S.; Haque, R. A.; Salman, A. W. Coord. Chem. Rev. 2012, 256, 1787.
|
|
|
(d) Gautam, A.; Shahini, C. R.; Siddappa, A. P.; Jan Grzegorz, M.; Hemavathi, B.; Ahipa, T. N.; Srinivasa, B. J. Organomet. Chem. 2021, 934, 121540.
|
|
|
(e) Astakhov, A. V.; Khazipov, O. V.; Chernenko, A. Y.; Pasyukov, D. V.; Kashin, A. S.; Gordeev, E. G.; Khrustalev, V. N.; Chernyshev, V. M.; Ananikov, V. P. Organometallics 2017, 36, 1981.
|
|
|
(f) Shao, H.; Ma, Y.; Lin, T.; Li, W.; Deng, Q. Asian J. Org. Chem. 2023, 12, e202300064.
|
|
|
(g) Sau, S. C.; Hota, P. K.; Mandal, S. K.; Soleilhavoup, M.; Bertrand, G. Chem. Soc. Rev. 2020, 49, 1233.
|
|
|
(h) Li, J.; He, D.; Lin, Z.; Wu, W.; Jiang, H. Org. Chem. Front. 2021, 8, 3502.
|
|
| [3] |
Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. J. Am. Chem. Soc. 2009, 131, 8344.
|
| [4] |
Altenhoff, G.; Goddard, R.; Lehmann, C. W.; Glorius, F. J. Am. Chem. Soc. 2004, 126, 15195.
pmid: 15548016 |
| [5] |
Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485.
|
| [6] |
Gyton, M. R.; Leforestier, B.; Chaplin, A. B. Organometallics 2018, 37, 3963.
|
| [7] |
(a) Luo, W.-Q.; Du, X.-G.; Chen, L.-Y.; Jin, C.-M. J. Organometal. Chem. 2021, 952, 122033.
|
|
(b) Peng, H. M.; Song, G.; Li, Y.; Li, X. Inorg. Chem. 2008, 47, 8031.
|
|
| [8] |
Wang, R.; Twamley, B.; Shreeve, J. M. J. Org. Chem. 2006, 71, 426.
|
| [9] |
Sie, M.-H.; Hsieh, Y.-H.; Tsai, Y.-H.; Wu, J.-R.; Chen, S.-J.; Lii, J.-H.; Lee, H. M. Organometallics 2010, 29, 6473.
|
| [10] |
Turek, J.; Panov, I.; Semler, M.; Štěpnička, P.; De Proft, F.; Padělková, Z.; Růžička, A. Organometallics 2014, 33, 3108.
|
| [11] |
(a) Ammar, H. B.; Hassine, B. B.; Fischmeister, C.; Dixneuf, P. H.; Bruneau, C. Eur. J. Inorg. Chem. 2010, 2010, 4752.
|
|
(b) Hoffmann, M.; Dagorne, S.; Pale, P.; Blanc, A.; de Frémont, P. J. Organomet. Chem. 2022, 979, 122507.
|
|
|
(c) Herrmann, W. A.; Goossen, L. J.; Spiegler, M. Organometallics 1998, 17, 2162.
|
|
| [12] |
(a) El Hatimi, A.; Gómez, M.; Jansat, S.; Muller, G.; Font-Bardía, M.; Solans, X. J. Chem. Soc., Dalton Trans. 1998, 4229.
|
|
(b) Viciano, M.; Feliz, M.; Corberán, R.; Mata, J. A.; Clot, E.; Peris, E. Organometallics 2007, 26, 5304.
|
|
|
(c) Wanniarachchi, Y. A.; Kogiso, Y.; Slaughter, L. M. Organometallics 2008, 27, 21.
|
|
| [13] |
Huang, S.; Hong, X.; Cui, H.-Z.; Zhan, B.; Li, Z.-M.; Hou, X.-F. Organometallics 2020, 39, 3514.
|
| [14] |
(a) Huang, S.; Wu, S.-P.; Zhou, Q.; Cui, H.-Z.; Hong, X.; Lin, Y.-J.; Hou, X.-F. J. Organomet. Chem. 2018, 868, 14.
|
|
(b) Huang, S.; Hong, X.; Cui, H.-Z.; Zhou, Q.; Lin, Y.-J.; Hou, X.-F. Dalton Trans. 2019, 48, 5072.
|
|
| [15] |
(a) Zhou, Q.; Hong, X.; Cui, H.-Z.; Huang, S.; Yi, Y.; Hou, X.-F. J. Org. Chem. 2018, 83, 6363.
|
|
(b) Zhou, Q.; Liu, S.; Ma, M.; Cui, H.-Z.; Hong, X.; Huang, S.; Zhang, J.-F.; Hou, X.-F. Synthesis 2018, 50, 1315.
|
|
|
(c) Shen, M. H.; Li, L. Q.; Zhou, Q.; Wang, J. H.; Wang, L. Chin. J. Org. Chem. 2023, 43, 697. (in Chinese)
|
|
|
(沈梦涵, 李来强, 周泉, 王洁慧, 王磊, 有机化学, 2023, 43, 697.)
doi: 10.6023/cjoc202207031 |
|
|
(d) Zhou, Q.; Yu, H. Y.; Zhou, Y. Q.; Wei, J. R.; Wang, L. Org. Biomol. Chem. 2022, 20, 5575.
|
|
| [16] |
Almy, J.; Martinez Alverez, R.; Fernandez, A. H.; Vazquez, A. S. J. Chem. Educ. 1997, 74, 1479.
|
| [17] |
Minghetti, G.; Cinellu, M. A.; Bandini, A. L.; Banditelli, G.; Demartin, F.; Manassero, M. J. Organometal. Chem. 1986, 315, 387.
|
| [18] |
Sugiyama, T.; Meng, J.; Matsuura, T. Acta Crystallogr.,Sect. C: Struct. Chem. 2002, 58, o242.
|
| [19] |
Chen, W.; Chen, K.; Chen, W.; Liu, M.; Wu, H. ACS Catal. 2019, 9, 8110.
doi: 10.1021/acscatal.9b02760 |
| [20] |
Zhou, J.; Luo, S.; Liu, H.; Xue, P. J. Organomet. Chem. 2022, 965-966, 122323.
|
| [21] |
Pauling, L. J. Chem. Educ. 1992, 69, 519.
|
| [22] |
Sugiyama, T.; Meng, J. B.; Matsuura, T. Acta Crystallogr., Sect. C: Struct. Chem. 2002, 58, 242.
|
| [23] |
Chakrabortty, S.; Kaur, M.; Adhikari, M.; Manar, K. K.; Singh, S. Inorg. Chem. 2021, 60, 6209.
|
| [24] |
(a) Pan, S.; Song, M.; Zuo, L.; Geng, X.; Wang, L. J. Org. Chem. 2023, 88, 5586.
|
|
(b) Xu, Z.; Geng, X.; Cai, Y.; Wang, L. J. Org. Chem. 2022, 87, 6562.
|
|
|
(c) Huo, J.; Geng, X.; Li, W.; Zhang, P.; Wang, L. Org. Lett. 2023, 25, 512.
|
|
|
(d) Zhang, J.-Q.; Hu, D.; Wang, J.; Ni, B.; Ren, H. Org. Lett. 2022, 24, 7905.
|
|
|
(e) Zhang, J.-Q.; Liu, J.; Hu, D.; Song, J.; Zhu, G.; Ren, H. Org. Lett. 2022, 24, 786.
|
|
|
(f) Hou, Z.-W.; Jiang, T.; Wu, T.-X.; Wang, L. Org. Lett. 2021, 23, 8585.
|
|
|
(g) Li, J.-S.; Liu, Y.-J.; Zhang, G.-W.; Ma, J.-A. Org. Lett. 2017, 19, 6364.
|
|
|
(h) Pan, Z.; Wang, X.; Zhao, S.; Deng, H.; Ma, M.; Xue, F. Org. Lett. 2023, 25, 6143.
|
|
|
(i) Sun, J.; Miao, T.; Li, P.; Wang, L. Chin. J. Org. Chem. 2021, 41, 3144. (in Chinese)
|
|
|
(孙佳兵, 苗涛, 李品华, 王磊, 有机化学, 2021, 41, 3144.)
doi: 10.6023/cjoc202102025 |
|
|
(j) Gao, R.; Zuo, L.; Wang, F.; Li, C.; Jiang, H.; Li, P.; Wang, L. Chin. J. Org. Chem. 2022, 42, 1883.
|
|
|
(k) He, H.; Lv, Y.; Hu, J.; Hou, Z.-W.; Wang, L. Green Chem. 2024, 26, 2157.
|
|
|
(l) Sun, C.; Zhou, Q.; Li, C.-Y.; Hou, Z.-W.; Wang, L. Org. Lett. 2024, 26, 883.
|
|
|
(m) Zhang, Z.; Zhou, Y.; Wang, J.; Zhang, Y.; Wang, L.; Liu, J.; Zhou, C.; Wang, M.; Li, P. Org. Biomol. Chem. 2024, 22, 1708.
|
|
|
(n) Hou, Z.-W.; Xu, H.-C.; Wang, L. Curr. Opin. Electrochem. 2024, 44, 101447.
|
|
| [25] |
Sakaguchi, H.; Uetake, Y.; Ohashi, M.; Niwa, T.; Ogoshi, S.; Hosoya, T. J. Am. Chem. Soc. 2017, 139, 12855.
doi: 10.1021/jacs.7b08343 pmid: 28849929 |
| [26] |
King, R. P.; Krska, S. W.; Buchwald, S. L. Org. Lett. 2021, 23, 7927.
|
| [27] |
(a) Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
|
|
(b) Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Acta Crystallogr.,Sect. A: Found. Adv. 2015, 71, 59.
|
|
| [28] |
Sheldrick, G. M. Acta Crystallogr.,Sect. C: Struct. Chem. 2015, 71, 3.
|
| [1] | 曹香雪, 贾雅会, 殷世纪, 徐亮, 韦玉, 宋欢欢. 可见光诱导二氢喹唑啉酮碳碳键断裂与三氟甲基取代烯烃的脱氟烷基化反应研究[J]. 有机化学, 2024, 44(5): 1549-1557. |
| [2] | 常哲, 王佳鑫, 陆熹, 傅尧. 镍促进电化学还原交叉偶联合成偕二氟烯烃[J]. 有机化学, 2022, 42(1): 147-159. |
| [3] | 张雷, 杨晨, 郭雪峰, 莫凡洋. Suzuki-Miyaura偶联反应机理研究进展[J]. 有机化学, 2021, 41(9): 3492-3510. |
| [4] | 李志清, 邱潇杨, 娄江, 王强. 可见光催化偕二氟烯烃碳-氟键官能化反应的研究进展[J]. 有机化学, 2021, 41(11): 4192-4207. |
| [5] | 王涛, 许凯, 孟团结, 张安安, 王红雨, 沈思思, 刘澜涛. 吖啶作为辅助配体的N-杂环卡宾-钯(Ⅱ)化合物:合成、表征和催化应用[J]. 有机化学, 2017, 37(7): 1794-1799. |
| [6] | 孟瑾, 杜利月, 郭留城. 苯基萘类木脂素内酯的化学合成[J]. 有机化学, 2016, 36(11): 2723-2728. |
| [7] | 陈旺, 郝慧琳, 张晨露, 沈月毛. Lycogarubin C和Lycogalic Acid的化学合成及抗肿瘤活性研究[J]. 有机化学, 2014, 34(4): 797-803. |
| [8] | 徐广庆, 赵庆, 汤文军. 发展高效的不对称Suzuki-Miyaura偶联反应及其合成应用[J]. 有机化学, 2014, 34(10): 1919-1940. |
| [9] | 陈国军, 杜建时. 镍催化酚衍生物的Suzuki-Miyaura偶联反应研究进展[J]. 有机化学, 2014, 34(1): 65-80. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||