有机化学 ›› 2011, Vol. 31 ›› Issue (10): 1690-1694. 上一篇 下一篇
研究简报
张红1,2,刘文杰2,曹德榕*,1,江焕峰1
Zhang Hong1,2 Liu Wenjie1 Cao Derong*,1 Jiang Huanfeng1
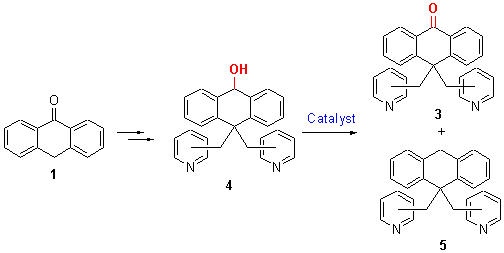
蒽酮1和氯甲基吡啶盐酸盐2在甲苯中回流反应生成10,10-二吡啶甲基-9(10H)蒽酮(3), 收率63%~68%|3用硼氢化钠还原生成10,10-二吡啶甲基-9,10-二氢蒽-9-醇(4), 收率87%~90%|蒽醇4在酸催化下发生歧化反应, 得到还原产物10,10-二吡啶甲基-9,10-二氢蒽(5)和氧化产物蒽酮3. 该歧化反应受催化剂、溶剂和反应温度等影响. 当蒽醇4用三氟化硼为催化剂、甲苯为溶剂、回流反应, 5的收率达到74%. 所合成的新化合物都经1H NMR, 13C NMR, MS和元素分析表征确认.