有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4327-4337.DOI: 10.6023/cjoc202106038 上一篇 下一篇
研究论文
王恒定a,b, 江凌a,b, 梁鸿雁c, 樊红军a,b,*( )
)
收稿日期:2021-06-22
修回日期:2021-08-04
发布日期:2021-08-24
通讯作者:
樊红军
基金资助:
Hengding Wanga,b, Ling Jianga,b, Hongyan Liangc, Hongjun Fana,b( )
)
Received:2021-06-22
Revised:2021-08-04
Published:2021-08-24
Contact:
Hongjun Fan
Supported by:文章分享

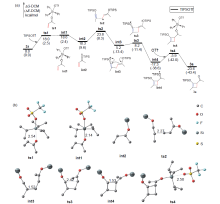
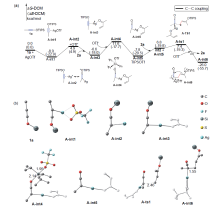
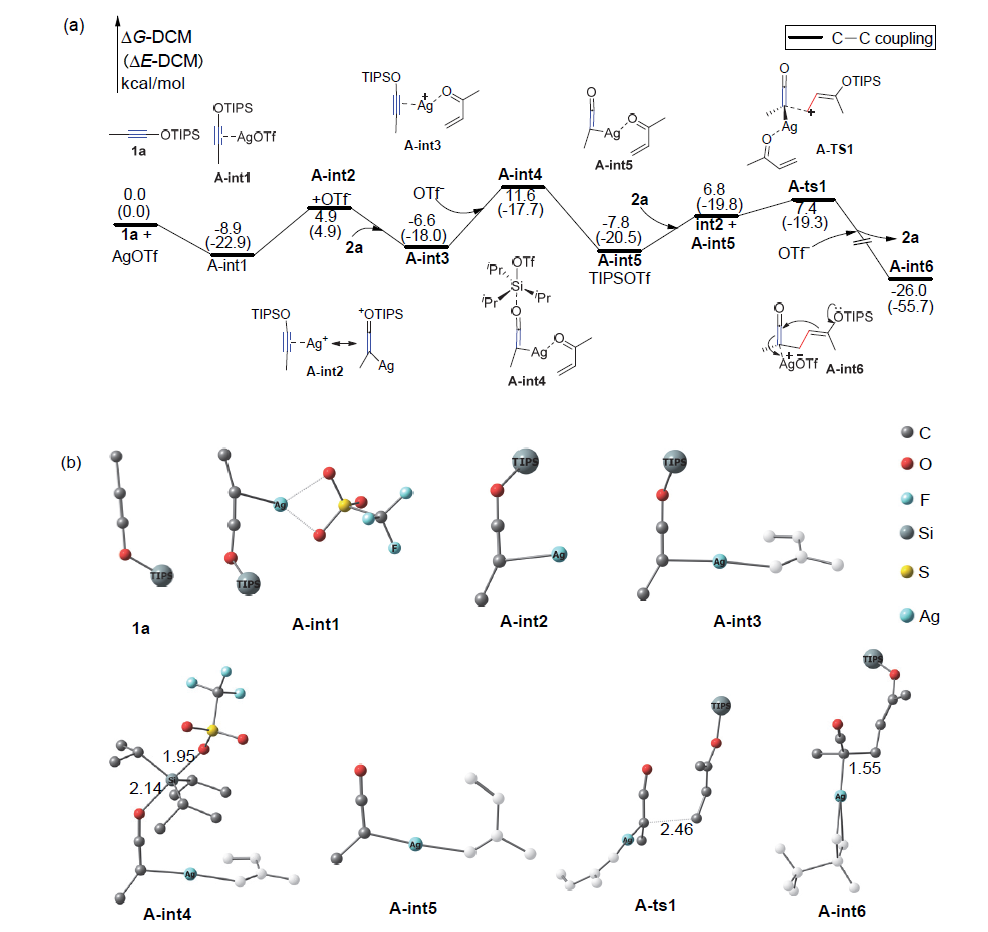
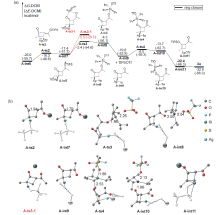
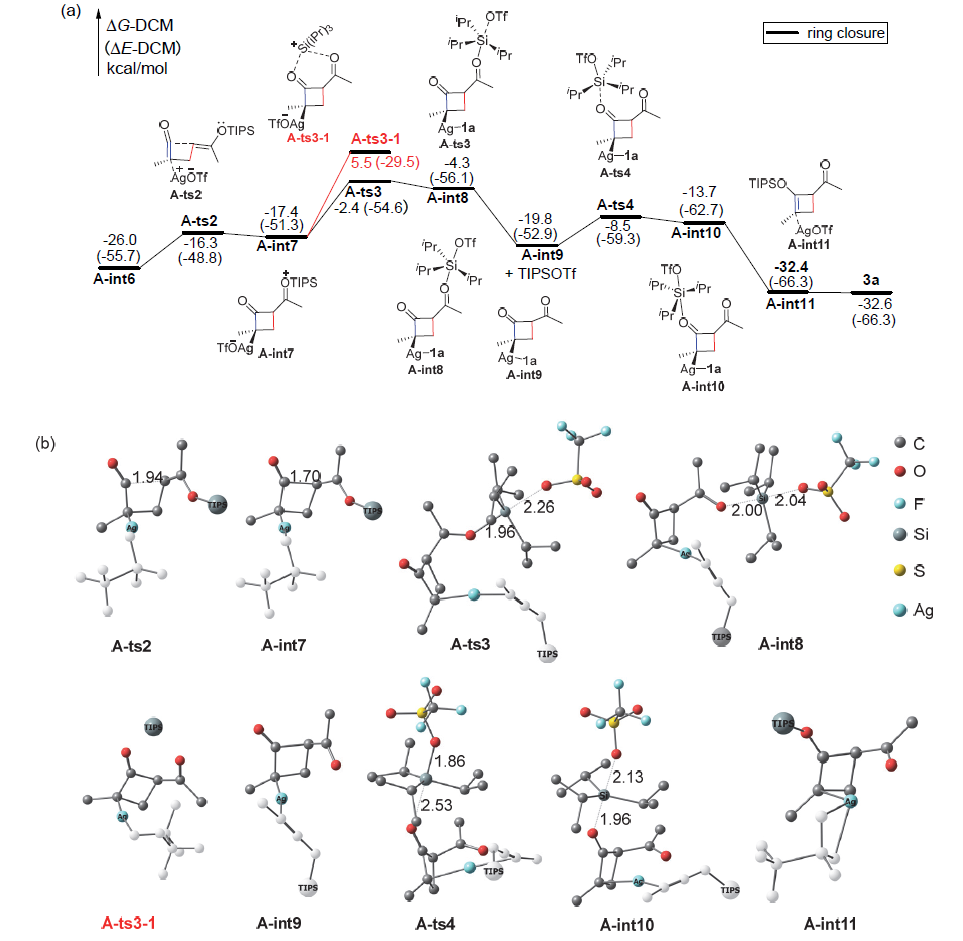
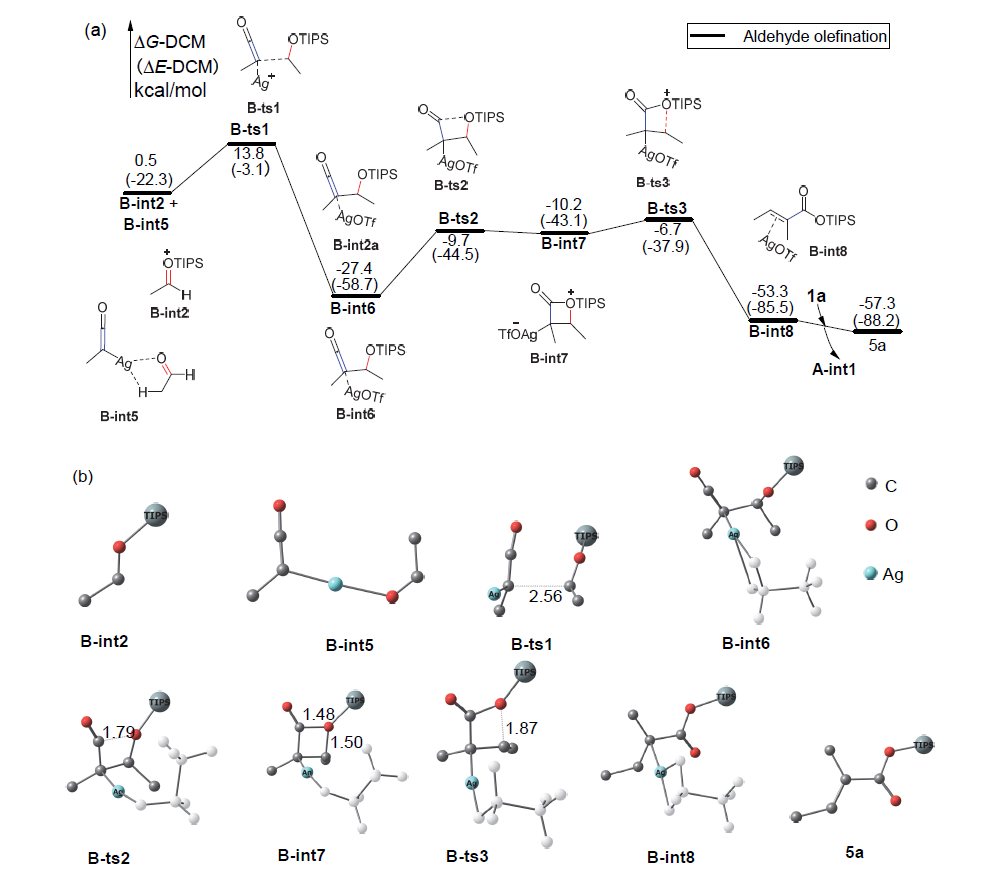
银离子可以高效地催化硅烷氧基炔与α,β-不饱和羰基化合物之间的[2+2]环加成反应, 在银离子催化下硅烷氧基炔可以与甲醛的羰基发生[2+2]环加成后开环生成不饱和酯. 利用密度泛函理论(DFT)计算发现, 在这两个反应中银离子与硅烷氧基炔之间相互作用, 将硅烷氧基炔激活成为硅正离子和银烯酮, 硅正离子进一步催化羰基化合物(α,β-不饱和羰基化合物或者醛)和银烯酮之间的[2+2]环加成反应.
王恒定, 江凌, 梁鸿雁, 樊红军. 银催化的硅烷氧基炔和羰基化合物的[2+2]环加成机理:硅正离子迁移主导的过程[J]. 有机化学, 2021, 41(11): 4327-4337.
Hengding Wang, Ling Jiang, Hongyan Liang, Hongjun Fan. Mechanism of Silver-Catalyzed [2+2] Cycloaddition between Siloxy-Alkynes and Carbonyl Compound: A Silylium Ion Migration Approach[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4327-4337.


| [1] |
Hoffmann, R.; Woodward, R. B. Acc. Chem. Res. 1968, 1, 17.
doi: 10.1021/ar50001a003 |
| [2] |
(a) Xu, C.-H.; Li, Y.; Li, J.-H.; Xiang, J.-N.; Deng, W. Sci. China: Chem. 2019, 62, 1463.
doi: 10.1007/s11425-018-9393-6 |
|
(b) Liu, Y.; Bandini, M. Chin. J. Chem. 2019, 37, 431.
doi: 10.1002/cjoc.v37.5 |
|
|
(c) Li, Y.; Jin, G.-F.; An, Y.-Y.; Das, R.; Han, Y.-F. Chin. J. Chem. 2019, 37, 1147.
doi: 10.1002/cjoc.v37.11 |
|
|
(d) Zhang, R.; Yuan, Y.; Qiu, Z.; Xie, Z. Chin. J. Chem. 2018, 36, 273.
doi: 10.1002/cjoc.v36.4 |
|
|
(e) Li, K.; Bai, L.; Luan, X. Chin. J. Org. Chem. 2019, 39, 2211. (in Chinese)
doi: 10.6023/cjoc201903065 |
|
|
(李锟雨, 白璐, 栾新军, 有机化学, 2019, 39, 2211.)
doi: 10.6023/cjoc201903065 |
|
|
(f) Li, Z.; Jian, H.; Wang, W.; Wang, Q.; He, L. Chin. J. Org. Chem. 2018, 38, 2045. (in Chinese)
doi: 10.6023/cjoc201802003 |
|
|
(李志娟, 翦辉, 王伟华, 王强, 何林, 有机化学, 2018, 38, 2045.)
doi: 10.6023/cjoc201802003 |
|
|
(g) Zhao, L.; Tong, X.; Zhu, H.; Yang, D.; Fan, M. Chin. J. Org. Chem. 2017, 37, 646. (in Chinese)
doi: 10.6023/cjoc201609014 |
|
|
(赵立芳, 同晓娟, 祝海涛, 杨得锁, 凡明锦, 有机化学, 2017, 37, 646.)
doi: 10.6023/cjoc201609014 |
|
| [3] |
Ohno, H.; Mizutani, T.; Kadoh, Y.; Miyamura, K.; Tanaka, T. Angew. Chem., Int. Ed. 2005, 44, 5113.
doi: 10.1002/(ISSN)1521-3773 |
| [4] |
Novikov, A. S. Inorg. Chim. Acta 2020, 510, 119758.
doi: 10.1016/j.ica.2020.119758 |
| [5] |
(a) Arnó, M.; Zaragozá, R. J.; Domingo, L. R. Eur. J. Org. Chem. 2005, 2005, 3973.
doi: 10.1002/(ISSN)1099-0690 |
|
(b) Domingo, L. R.; Ríos-Gutiérrez, M.; Silvi, B.; Pérez, P. Eur. J. Org. Chem. 2018, 2018, 1107.
|
|
|
(c) Mu, B.-S.; Zhang, Z.-H.; Wu, W.-B.; Yu, J.-S.; Zhou, J. Acta Chim. Sinica 2021, 79, 685. (in Chinese)
doi: 10.6023/A21040131 |
|
|
(穆博帅, 张志豪, 武文彪, 余金生, 周剑, 化学学报, 2021, 79, 685.)
doi: 10.6023/A21040131 |
|
|
(d) Lu, P.; Ma, S. Chin. J. Chem. 2010, 28, 1600.
doi: 10.1002/cjoc.201090271 |
|
| [6] |
Xu, Y.; Conner, M. L.; Brown, M. K. Angew. Chem., Int. Ed. 2015, 54, 11918.
doi: 10.1002/anie.201502815 |
| [7] |
(a) Sarkar, D.; Bera, N.; Ghosh, S. Eur. J. Org. Chem. 2020, 2020, 1310.
doi: 10.1002/ejoc.201901143 pmid: 27018601 |
|
(b) Poplata, S.; Troster, A.; Zou, Y. Q.; Bach, T. Chem. Rev. 2016, 116, 9748.
doi: 10.1021/acs.chemrev.5b00723 pmid: 27018601 |
|
|
(c) Singh, K.; Trinh, W.; Weaver, J. D. III. Org. Biomol. Chem. 2019, 17, 1854.
doi: 10.1039/C8OB01273C pmid: 27018601 |
|
|
(d) Gan, M.; Zhang, W.; Huo, X.; Han, Y. Sci. Sin.: Chim. 2017, 47, 705. (in Chinese)
pmid: 27018601 |
|
|
(甘明明, 张伟, 霍现宽, 韩英锋, 中国科学: 化学, 2017, 47, 705.)
pmid: 27018601 |
|
| [8] |
(a) Ren, X.; Lu, Y.; Lu, G.; Wang, Z. X. Org. Lett. 2020, 22, 2454.
doi: 10.1021/acs.orglett.0c00674 |
|
(b) Zhao, L.; Zhang, L.; Fang, D.-C. Organometallics 2016, 35, 3577.
doi: 10.1021/acs.organomet.6b00646 |
|
|
(c) Canellas, S.; Montgomery, J.; Pericas, M. A. J. Am. Chem. Soc. 2018, 140, 17349.
doi: 10.1021/jacs.8b09677 |
|
|
(d) Hoyt, J. M.; Schmidt, V. A.; Tondreau, A. M.; Chirik, P. J. Science 2015, 349, 960.
doi: 10.1126/science.aac7440 |
|
|
(e) Zeng, Z.; Yang, D. Chin. J. Org. Chem. 2013, 33, 2131. (in Chinese)
doi: 10.6023/cjoc201301072 |
|
|
(曾中一, 杨定乔, 有机化学, 2013, 33, 2131.)
doi: 10.6023/cjoc201301072 |
|
| [9] |
(a) Boxer, M. B.; Yamamoto, H. Org. Lett. 2005, 7, 3127.
doi: 10.1021/ol0512334 pmid: 15771480 |
|
(b) Inanaga, K.; Takasu, K.; Ihara, M. J. Am. Chem. Soc. 2005, 127, 3668.
pmid: 15771480 |
|
|
(c) Shen, L.; Zhao, K.; Doitomi, K.; Ganguly, R.; Li, Y. X.; Shen, Z. L.; Hirao, H.; Loh, T. P. J. Am. Chem. Soc. 2017, 139, 13570.
doi: 10.1021/jacs.7b07997 pmid: 15771480 |
|
| [10] |
Takasu, K.; Ueno, M.; Inanaga, K.; Ihara, M. J. Org. Chem. 2004, 69, 517.
doi: 10.1021/jo034989u |
| [11] |
(a) Muratore, M. E.; Homs, A.; Obradors, C.; Echavarren, A. M. Chem.-Asian J. 2014, 9, 3066.
doi: 10.1002/asia.201402395 |
|
(b) Zhou, L.; Xu, B.; Ji, D.; Zhang, Z.-M.; Zhang, J. Chin. J. Chem. 2020, 38, 577.
doi: 10.1002/cjoc.v38.6 |
|
|
(c) Zhou, Y.; Liu, S.; Chen, H.; Chen, J.; Sun, W.; Li, S.; Yang, Q.; Fan, B. Chin. J. Chem. 2015, 33, 1115.
doi: 10.1002/cjoc.201500392 |
|
| [12] |
Sweis, R. F.; Schramm, M. P.; Kozmin, S. A. J. Am. Chem. Soc. 2004, 126, 7442.
doi: 10.1021/ja048251l |
| [13] |
Sumaria, C. S.; Turkmen, Y. E.; Rawal, V. H. Org. Lett. 2014, 16, 3236.
doi: 10.1021/ol501254h |
| [14] |
Sun, J.; Keller, V. A.; Meyer, S. T.; Kozmin, S. A. Adv. Synth. Catal. 2010, 352, 839.
doi: 10.1002/adsc.200900835 |
| [15] |
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. RSC Adv. 2020, 10, 15394.
doi: 10.1039/D0RA01548B |
| [16] |
Wang, H.-D.; Fan, H.-J. Commun. Chem. 2020, 3, 126.
doi: 10.1038/s42004-020-00373-2 |
| [17] |
Turkmen, Y. E.; Montavon, T. J.; Kozmin, S. A.; Rawal, V. H. J. Am. Chem. Soc. 2012, 134, 9062.
doi: 10.1021/ja302537j |
| [18] |
Mueller, T. In Functional Molecular Silicon Compounds I: Regular Oxidation States, 155, Eds.: Scheschkewitz, D., Springer, New York, 2014, p. 107.
|
| [19] |
Fang, G.; Bi, X. Chem. Soc. Rev. 2015, 44, 8124.
doi: 10.1039/C5CS00027K |
| [20] |
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
doi: 10.1002/jcc.v33.5 |
| [21] |
Johnson, E. R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A. J.; Yang, W. J. Am. Chem. Soc. 2010, 132, 6498.
doi: 10.1021/ja100936w pmid: 20394428 |
| [22] |
Lu, T.; Chen, Q. Chem. Methods 2021, 1, 231.
doi: 10.1002/cmtd.v1.5 |
| [23] |
Xiao, P.; Li, C. X.; Fang, W. H.; Cui, G.; Thiel, W. J. Am. Chem. Soc. 2018, 140, 15099.
doi: 10.1021/jacs.8b10387 pmid: 30362731 |
| [24] |
Domingo, L. R.; Rios-Gutierrez, M.; Perez, P. Molecules 2016, 21, 748.
doi: 10.3390/molecules21060748 |
| [25] |
Allen, A. D.; Tidwell, T. T. In Advances in Physical Organic Chemistry, Vol. 48, Eds.: Ian, N. H. W.; Williams, H., Elsevier Ltd., UK, 2014, p. 229.
|
| [26] |
Antoniotti, S.; Dalla, V.; Dunach, E. Angew. Chem., Int. Ed. 2010, 49, 7860.
doi: 10.1002/anie.v49:43 |
| [27] |
(a) Kazakova, A. N.; Vasilyev, A. V. Russ. J. Org. Chem. 2017, 53, 485.
doi: 10.1134/S1070428017040017 |
|
(b) Greb, L. Chem.-Eur. J. 2018, 24, 17881.
doi: 10.1002/chem.v24.68 |
|
| [28] |
(a) Shindo, M.; Matsumoto, K. In Stereoselective Alkene Synthesis, Vol. 327, Ed.: Wang, J., Springer, New York, 2012, p. 1.
|
|
(b) Shindo, M. Synthesis-Stuttgart 2003, 15, 2275.
|
|
|
(c) Qian, H.; Zhao, W.; Sun, J. Chem. Rec. 2014, 14, 1070.
doi: 10.1002/tcr.v14.6 |
|
| [29] |
Allen, A. D.; Tidwell, T. T. ARKIVOC 2016, i, 415.
|
| [30] |
Huang, L.; Wu, J.; Hu, J.; Bi, Y.; Huang, D. Tetrahedron Lett. 2020, 61, 151363.
doi: 10.1016/j.tetlet.2019.151363 |
| [31] |
Lee, V. Y. Russ. Chem. Rev. 2019, 88, 351.
doi: 10.1070/RCR4868 |
| [32] |
(a) Lambert, J. B.; Zhang, S. H.; Stern, C. L.; Huffman, J. C. Science 1993, 260, 1917.
pmid: 21527709 |
|
(b) Kim, K. C.; Reed, C. A.; Elliott, D. W.; Mueller, L. J.; Tham, F.; Lin, L. J.; Lambert, J. B. Science 2002, 297, 825.
doi: 10.1126/science.1073540 pmid: 21527709 |
|
|
(c) Douvris, C.; Ozerov, O. V. Science 2008, 321, 1188.
doi: 10.1126/science.1159979 pmid: 21527709 |
|
|
(d) Allemann, O.; Duttwyler, S.; Romanato, P.; Baldridge, K. K.; Siegel, J. S. Science 2011, 332, 574.
doi: 10.1126/science.1202432 pmid: 21527709 |
|
| [33] |
Avcı, Ö. N.; Catak, S.; Dereli, B.; Aviyente, V.; Dedeoglu, B. ChemCatChem 2019, 12, 366.
doi: 10.1002/cctc.v12.1 |
| [34] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J.,Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford, CT, 2016.
|
| [35] |
Chemcraft-graphical software for visualization of quantum chemistry computations. https://www.chemcraftprog.com.
|
| [36] |
Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615.
doi: 10.1039/b810189b |
| [37] |
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
doi: 10.1039/b508541a |
| [38] |
Fukui, K. Acc. Chem. Res. 1981, 14, 363.
doi: 10.1021/ar00072a001 |
| [39] |
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
doi: 10.1021/jp810292n |
| [40] |
(a) Ryu, H.; Park, J.; Kim, H. K.; Park, J. Y.; Kim, S.-T.; Baik, M.-H. Organometallics 2018, 37, 3228.
doi: 10.1021/acs.organomet.8b00456 |
|
(b) Wei, C. S.; Jiménez-Hoyos, C. A.; Videa, M. F.; Hartwig, J. F.; Hall, M. B. J. Am. Chem. Soc. 2010, 132, 3078.
doi: 10.1021/ja909453g |
| [1] | 李文娟, 张睿, 蔡志华, 韩小强, 何林, 代斌. 苯炔[3+2]环加成反应构建三氟甲基取代的苯并环状亚砜亚胺衍生物及其杀棉蚜活性研究[J]. 有机化学, 2022, 42(9): 2832-2839. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||