有机化学 ›› 2022, Vol. 42 ›› Issue (6): 1799-1810.DOI: 10.6023/cjoc202111038 上一篇 下一篇
研究论文
王朝彧, 董书达, 朱天阳, 刘玉琴, 武梓涵, 冯若昆*( )
)
收稿日期:2021-11-25
修回日期:2022-01-14
发布日期:2022-02-17
通讯作者:
冯若昆
基金资助:
Chaoyu Wang, Shuda Dong, Tianyang Zhu, Yuqin Liu, Zihan Wu, Ruokun Feng( )
)
Received:2021-11-25
Revised:2022-01-14
Published:2022-02-17
Contact:
Ruokun Feng
Supported by:文章分享
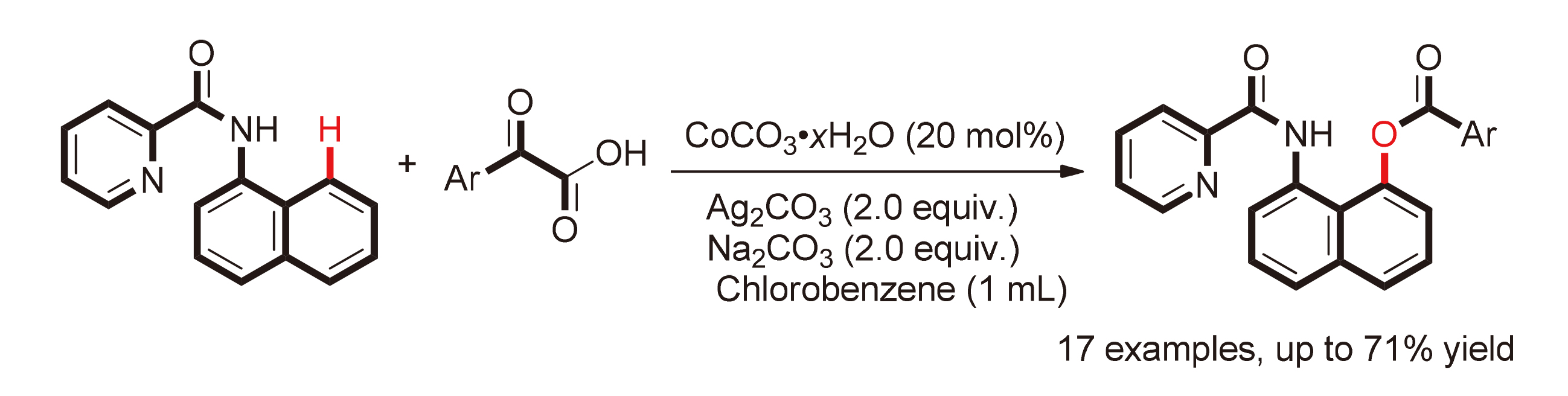
研究了碱式碳酸钴为催化剂, 碳酸银为氧化剂, 碳酸钠为碱, 1-萘胺衍生物与α-羰基羧酸的sp2碳氢键活化酰氧基化反应, 从而得到了相应的芳香酯类化合物. 各种取代的苯甲酰甲酸、萘甲酰甲酸及噻吩-2-甲酰甲酸均能以中等及以上的收率得到目标化合物. 此外, 当反应中使用氘代苯甲酰甲酸作为反应底物时, 反应仍可以以62%的产率得到同位素标记的芳香酯化产物.
王朝彧, 董书达, 朱天阳, 刘玉琴, 武梓涵, 冯若昆. 钴催化的1-萘胺衍生物与α-羰基羧酸的脱羰C(8)-位酰氧基化反应[J]. 有机化学, 2022, 42(6): 1799-1810.
Chaoyu Wang, Shuda Dong, Tianyang Zhu, Yuqin Liu, Zihan Wu, Ruokun Feng. Cobalt-Catalyzed Decarbonylative C(8)-Acyloxylation of 1-Naphthalamine Derivatives with α-Oxocarboxylic Acids[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1799-1810.
| Entry | Catalyst | Oxidant | Base | Solvent | Yieidb/% |
|---|---|---|---|---|---|
| 1 | Co(OAc)2 | Ag2CO3 | Na2CO3 | Chlorobenzene | 18 |
| 2 | CoBr2 | Ag2CO3 | Na2CO3 | Chlorobenzene | 15 |
| 3 | CoO | Ag2CO3 | Na2CO3 | Chlorobenzene | N.R. |
| 4 | CoSO4•7H2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 16 |
| 5 | Co2(CO)8 | Ag2CO3 | Na2CO3 | Chlorobenzene | Trace |
| 6 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 71 |
| 7 | [Cp*Co(CO)I2] | Ag2CO3 | Na2CO3 | Chlorobenzene | 21 |
| 8 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | o-Chlorotoluene | 53 |
| 9 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Fluorobenzene | Trace |
| 10 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Toluene | 26 |
| 11 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Nitrobenzene | 17 |
| 12 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Dimethylbenzene | 40 |
| 13 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | m-Xylene | 41 |
| 14 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | 1,2-Dichloroethane | Trace |
| 15 | CoCO3•xH2O | AgNO3 | Na2CO3 | Chlorobenzene | 29 |
| 16 | CoCO3•xH2O | Ag2O | Na2CO3 | Chlorobenzene | 61 |
| 17 | CoCO3•xH2O | Mn(OAc)2 | Na2CO3 | Chlorobenzene | Trace |
| 18 | CoCO3•xH2O | Ag2CO3 | NaHCO3 | Chlorobenzene | 35 |
| 19 | CoCO3•xH2O | Ag2CO3 | KHCO3 | Chlorobenzene | 56 |
| 20 | CoCO3•xH2O | Ag2CO3 | Cs2CO3 | Chlorobenzene | N.R. |
| 21 | CoCO3•xH2O | Ag2CO3 | DBU | Chlorobenzene | N.R. |
| 22 | — | Ag2CO3 | Na2CO3 | Chlorobenzene | N.R |
| 23 | CoCO3•xH2O | — | Na2CO3 | Chlorobenzene | N.R |
| 24 | CoCO3•xH2O | Ag2CO3 | — | Chlorobenzene | N.R |
| 25 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 51c |
| 26 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 39d |
| 27 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 49e |
| 28 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 61f |
| 29 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | Traceg |
| Entry | Catalyst | Oxidant | Base | Solvent | Yieidb/% |
|---|---|---|---|---|---|
| 1 | Co(OAc)2 | Ag2CO3 | Na2CO3 | Chlorobenzene | 18 |
| 2 | CoBr2 | Ag2CO3 | Na2CO3 | Chlorobenzene | 15 |
| 3 | CoO | Ag2CO3 | Na2CO3 | Chlorobenzene | N.R. |
| 4 | CoSO4•7H2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 16 |
| 5 | Co2(CO)8 | Ag2CO3 | Na2CO3 | Chlorobenzene | Trace |
| 6 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 71 |
| 7 | [Cp*Co(CO)I2] | Ag2CO3 | Na2CO3 | Chlorobenzene | 21 |
| 8 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | o-Chlorotoluene | 53 |
| 9 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Fluorobenzene | Trace |
| 10 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Toluene | 26 |
| 11 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Nitrobenzene | 17 |
| 12 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Dimethylbenzene | 40 |
| 13 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | m-Xylene | 41 |
| 14 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | 1,2-Dichloroethane | Trace |
| 15 | CoCO3•xH2O | AgNO3 | Na2CO3 | Chlorobenzene | 29 |
| 16 | CoCO3•xH2O | Ag2O | Na2CO3 | Chlorobenzene | 61 |
| 17 | CoCO3•xH2O | Mn(OAc)2 | Na2CO3 | Chlorobenzene | Trace |
| 18 | CoCO3•xH2O | Ag2CO3 | NaHCO3 | Chlorobenzene | 35 |
| 19 | CoCO3•xH2O | Ag2CO3 | KHCO3 | Chlorobenzene | 56 |
| 20 | CoCO3•xH2O | Ag2CO3 | Cs2CO3 | Chlorobenzene | N.R. |
| 21 | CoCO3•xH2O | Ag2CO3 | DBU | Chlorobenzene | N.R. |
| 22 | — | Ag2CO3 | Na2CO3 | Chlorobenzene | N.R |
| 23 | CoCO3•xH2O | — | Na2CO3 | Chlorobenzene | N.R |
| 24 | CoCO3•xH2O | Ag2CO3 | — | Chlorobenzene | N.R |
| 25 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 51c |
| 26 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 39d |
| 27 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 49e |
| 28 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | 61f |
| 29 | CoCO3•xH2O | Ag2CO3 | Na2CO3 | Chlorobenzene | Traceg |
| [1] |
(a) Durward, W.; Cruickshank, J.; Sparks, R. A. Proc. R. Soc. London. Ser. A 1960, 258, 270.
|
|
(b) Prévost, S. ChemPlusChem 2020, 85, 476.
doi: 10.1002/cplu.202000005 |
|
| [2] |
O'Reilly, M.; Kirkwood, N. K.; Kenyon, E. J.; Huckvale, R.; Cantillon, D. M.; Waddell, S. J.; Ward, S. E.; Richardson, G. P.; Kros, C. J.; Derudas, M. J. Med. Chem. 2019, 62, 5312.
doi: 10.1021/acs.jmedchem.8b01325 pmid: 31083995 |
| [3] |
Panchgalle, S. P.; Gore, R. G.; Chavan, S. P.; Kalkote, U. R. Tetrahedron 2009, 20, 1767.
|
| [4] |
Example for Recent Advances in Directing Group-Induced C-H Activation Reactions, see: (a) Ujwaldev, S. M.; Harry, N. A.; Divakar, M. A.; Anilkumar, G. Catal. Sci. Technol. 2018, 8, 5983.
doi: 10.1039/C8CY01418C |
|
(b) Wang, S.; Yan, F.; Wang, L.; Zhu, L. Chin. J. Org. Chem. 2018, 38, 291. (in Chinese)
doi: 10.6023/cjoc201708055 |
|
|
汪珊, 严沣, 汪连生, 朱磊, 有机化学, 2018, 38, 291.).
doi: 10.6023/cjoc201708055 |
|
|
(c) Guan, Z.-H.; Usman, M.; Ren, Z.-H.; Wang, Y.-Y. Synthesis 2017, 49, 1419.
doi: 10.1055/s-0036-1589478 |
|
| [5] |
For selected papers on C-2 functionalizations of 1-naphthylamide derivatives, see: (a) Daugulis, O.; Zaitsev, V. G. Angew. Chem., Int. Ed. 2005, 44, 4046.
doi: 10.1002/anie.200500589 pmid: 25203435 |
|
(b) Kim, B. S.; Jang, C.; Lee, D. J.; Youn, S. W. Chem. Asian J. 2010, 5, 2336.
doi: 10.1002/asia.201000613 pmid: 25203435 |
|
|
(c) Yip, K.-T.; Yang, D. Org. Lett. 2011, 13, 2134.
doi: 10.1021/ol2006083 pmid: 25203435 |
|
|
(d) Wu, Y.; Choy, P. Y.; Mao, F.; Kwong, F. Y. Chem. Commun. 2013, 49, 689.
doi: 10.1039/C2CC37352A pmid: 25203435 |
|
|
(e) Szabó, F.; Daru, J.; Simkó, D.; Nagy, T. Z.; Stirling, A.; Novák, Z. Adv. Synth. Catal. 2013, 355, 685.
doi: 10.1002/adsc.201200948 pmid: 25203435 |
|
|
(f) Gao, Y.; Huang, Y.; Wu, W.; Huang, K.; Jiang, H. Chem. Commun. 2014, 50, 8370.
doi: 10.1039/c4cc03062a pmid: 25203435 |
|
|
(g) Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W. A.; Chen, G. Org. Lett. 2014, 16, 1764.
doi: 10.1021/ol500464x pmid: 25203435 |
|
|
(h) Iwasaki, M.; Iyanaga, M.; Tsuchiya, Y.; Nishimura, Y.; Li, W.; Li, Z.; Nishihara, Y. Chem.-Eur. J. 2014, 20, 2459.
doi: 10.1002/chem.201304717 pmid: 25203435 |
|
|
(h) Zhang, X.; Si, W.; Bao, M.; Asao, N.; Yamamoto, Y.; Jin, T. Org. Lett. 2014, 16, 4830.
doi: 10.1021/ol502317c pmid: 25203435 |
|
|
(i) Das, R.; Kapur, M. J. Org. Chem. 2017, 82, 1114.
doi: 10.1021/acs.joc.6b02731 pmid: 25203435 |
|
|
(j) Li, Z.-L.; Sun, K.-K.; Cai, C. Org. Biomol. Chem. 2018, 16, 5433.
doi: 10.1039/C8OB01448E pmid: 25203435 |
|
|
(k) Wu, P.; Huang, W.; Cheng, T.-J.; Lin, H.-X.; Dai, H.-X. Org. Lett. 2020, 22, 5051.
doi: 10.1021/acs.orglett.0c01632 pmid: 25203435 |
|
|
(l) Li, Q.; Huang, J.; Chen, G.; Wang, S.-B. Org. Biomol. Chem. 2020, 18, 4802.
doi: 10.1039/D0OB00784F pmid: 25203435 |
|
|
(m) Sarkar, S.; Sahoo, T.; Sen, C.; Ghosh, S. C. Chem. Commun. 2021, 57, 8949.
doi: 10.1039/D1CC01803E pmid: 25203435 |
|
|
(n) Wang, D.; Xu, X.; Zhang, J.; Zhao, Y. J. Org. Chem. 2021, 86, 2696.
doi: 10.1021/acs.joc.0c02701 pmid: 25203435 |
|
| [6] |
For selected papers on C-4-functionalizations of 1-naphthylamide derivatives, see: (a) Li, J.-M.; Wang, Y.-H.; Yu, Y.; Wu, R.-B.; Weng, J.; Lu, G. ACS Catal. 2017, 7, 2661.
doi: 10.1021/acscatal.6b03671 |
|
(b) Liang, S.; Bolte, M.; Manolikakes, G. Chem.-Eur. J. 2017, 23, 96.
doi: 10.1002/chem.201605101 |
|
|
(c) Bai, P.; Sun, S.; Li, Z.; Qiao, H.; Su, X.; Yang, F.; Wu, Y. Wu, Y. J. Org. Chem. 2017, 82, 12119.
doi: 10.1021/acs.joc.7b01917 |
|
|
(d) Han, S.; Liang, A.; Ren, X.; Gao, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2017, 58, 4859.
|
|
|
(e) You, G.; Wang, K.; Wang, X.; Wang, G.; Sun, J.; Duan, G.; Xia, C. Org. Lett. 2018, 20, 4005.
doi: 10.1021/acs.orglett.8b01395 |
|
|
(f) Zhu, H.; Sun, S.; Qiao, H.; Yang, F.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2018, 20, 620.
doi: 10.1021/acs.orglett.7b03749 |
|
|
(g) Dou, Y.; Yin, B.; Zhang, P.; Zhu, Q. Eur. J. Org. Chem. 2018, 4571.
|
|
|
(h) Xu, J.; Du, K.; Shen, J.; Shen, C.; Chai, K.; Zhang, P. ChemCatChem 2018, 10, 3675.
doi: 10.1002/cctc.201800328 |
|
|
(i) Zhao, L.; Sun, M.; Yang, F,; Wu, Y. J. Org. Chem. 2021, 86, 11519.
doi: 10.1021/acs.joc.1c00971 |
|
| [7] |
Example for Precious metal catalyzed C-8-functionalizations of 1-naphthylamide derivativesl, see: (a) Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78, 3030.
doi: 10.1021/jo400017v |
|
(b) Huang, L.; Sun, X.; Li, Q.; Qi, C. J. Org. Chem. 2014, 79, 6720.
|
|
|
(c) Iwasaki, M.; Kaneshika, W.; Tsuchiya, Y. J. Org. Chem. 2014, 79, 11330.
doi: 10.1021/jo502274t |
|
|
(d) Li, Z.; Sun, S.; Qiao, H.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2016, 18, 4594.
doi: 10.1021/acs.orglett.6b02243 |
|
|
(e) Zhu, H.; Sun, S.; Qiao, H.; Yang, F.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2018, 20, 620.
doi: 10.1021/acs.orglett.7b03749 |
|
|
(f) Han, J.-N.; Du, C.; Zhu, X.; Wang, Z.-L.; Zhu, Y.; Chu, Z.-Y.; Niu, J.-L.; Song, M.-P. Beilstein J. Org. Chem. 2018, 14, 2090.
doi: 10.3762/bjoc.14.183 |
|
|
(g) Yu, X.; Yang, F.; Wu, Y.; Wu, Y. Org. Lett. 2019, 21, 1726.
doi: 10.1021/acs.orglett.9b00283 |
|
|
(h) Zhang, T.; Zhu, H.; Yang, F.; Wu, Y.; Wu, Y. Tetrahedron 2019, 75, 1541.
doi: 10.1016/j.tet.2019.02.002 |
|
|
(i) Zhang, M.; Li, R.; Yang, Z.; Feng, R. Chin. J. Org. Chem. 2020, 40, 714. (in Chinese)
doi: 10.6023/cjoc201908040 |
|
|
( 张梦帆, 李瑞鹏, 杨震, 冯若昆, 有机化学, 2020, 40, 714.)
doi: 10.6023/cjoc201908040 |
|
|
(j) Gao, Y.; Zhang, M.; Wang, C.; Yang, Z.; Huang, X.; Feng, R.; Qi, C. Chem. Commun. 2020, 56, 14231.
doi: 10.1039/D0CC05616B |
|
|
(k) Zu, C.; Zhang, T.; Yang, F.; Wu, Y.; Wu, Y. J. Org. Chem. 2020, 85, 12777.
doi: 10.1021/acs.joc.0c01672 |
|
|
(l) Shi, Y.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2020, 18, 4628.
doi: 10.1039/D0OB00586J |
|
|
(m) Zu, C.; Zhang, T.; Yang, F.; Wu, Y.; Wu, Y. J. Org. Chem. 2020, 85, 12777.
doi: 10.1021/acs.joc.0c01672 |
|
|
(n) Sun, Y.; Feng, C.; Wang, P.; Yang, F.; Wu, Y. Org. Chem. Front. 2021, 8, 5710.
doi: 10.1039/D1QO00975C |
|
| [8] |
Recent reviews for Cross-Dehydrogenative Couplings CDC, see: (a) Zhang, Y.; Feng, B. Chin. J. Org. Chem. 2014, 34, 2406. (in Chinese)
doi: 10.6023/cjoc201408030 pmid: 25670997 |
|
( 张艳, 冯柏年, 有机化学, 2014, 34, 2406.)
doi: 10.6023/cjoc201408030 pmid: 25670997 |
|
|
(b) Krylov, I. B.; Vil’, V. A.; Terent'ev, A. O. Beilstein J. Org. Chem. 2015, 11, 92.
doi: 10.3762/bjoc.11.13 pmid: 25670997 |
|
|
(c) Arshadi, S.; Banaei, A.; Monfared, A.; Ebrahimiaslbc, S.; Hosseinian, A. RSC Adv. 2019, 9, 17101.
doi: 10.1039/c9ra01941c pmid: 25670997 |
|
|
(d) Peng, W.; Vessally, E.; Arshadi, S.; Monfared, A.; Hosseinian, A.; Edjlali, L. Top. Curr. Chem. 2019, 377, 20.
pmid: 25670997 |
|
|
(e) Peng, K.; Dong, Z. Adv. Synth. Catal. 2021, 363, 1185.
doi: 10.1002/adsc.202001358 pmid: 25670997 |
|
|
(f) Afsina, C. M. A.; Aneeja, T.; Neetha, M.; Anikumar, G. Eur. J. Org. Chem. 2021, 1776.
pmid: 25670997 |
|
| [9] |
Raghuvanshi, K.; Zell, D.; Ackermann, L. Org. Lett. 2017, 19, 1278.
doi: 10.1021/acs.orglett.6b03898 pmid: 28234011 |
| [10] |
Ye, Z.; Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Org. Lett. 2009, 11, 3974.
doi: 10.1021/ol901609t |
| [11] |
Wu, Y.; Zhou, B. Org. Lett. 2017, 1, 3532.
|
| [12] |
Lin, C.; Chen, Z.; Liu, Z.; Zhang, Y. Adv. Synth. Catal. 2018, 360, 519.
doi: 10.1002/adsc.201701144 |
| [13] |
Ueno, R.; Natsui, S.; Chatani, N. Org. Lett. 2018, 20, 1062.
doi: 10.1021/acs.orglett.7b04020 |
| [14] |
Lan, J.; Xie, H.; Lu, X.; Deng, Y.; Jiang, H.; Zeng, W. Org. Lett. 2017, 19, 4279.
doi: 10.1021/acs.orglett.7b01942 |
| [15] |
Example for preparation of aryl ketones through decarboxylative cross-coupling, see: (a) Guo, L.-N.; Wang, H.; Duan, X.-H. Org. Biol. Chem. 2016, 14, 7380.
doi: 10.1039/C6OB01113F |
|
(b) Huang, F.; Chen, X.; Xie, Y.; Zeng, W. Chin. J. Org. Chem. 2017, 37, 3. (in Chinese)
|
|
|
( 黄房生, 陈训, 谢应, 曾伟, 有机化学, 2017, 37, 31.)
doi: 10.6023/cjoc201607003 |
|
|
(c) Ruan, L.; Chen, C.; Zhang, X.; Jing, S. Chin. J. Org. Chem. 2018, 38, 3155. (in Chinese)
doi: 10.6023/cjoc201806009 |
|
|
( 阮利衡, 陈春欣, 张晓欣, 孙京, 有机化学, 2018, 38, 3155.)
doi: 10.6023/cjoc201806009 |
|
|
(d) Bao, P.; Liu, F.; Lv, Y.; Yue, H.; Li, J.; Wei, W. Org. Chem. Front. 2020, 7, 492.
doi: 10.1039/C9QO01334B |
|
|
(e) Lalji, R.; Kumar, P.; Gupta, M.; Parmar, V.; Singh, B. Adv. Synth. Catal. 2020, 362, 552.
doi: 10.1002/adsc.201901142 |
|
|
(f) Ni, H.; Shi, X.; Li, Y.; Zhang, X.; Zhao, J.; Zhao, F. Org. Biol. Chem. 2020, 18, 6558.
doi: 10.1039/D0OB01423K |
|
|
(g) Waghmare, D.-S.; Tambe, S.-D.; Kshirsagar, U.-A. Asian J. Org. Chem. 2020, 9, 2095.
doi: 10.1002/ajoc.202000487 |
|
|
(h) Hou, C.; Sun, S.; Liu, Z.; Zhang, H.; Liu, Y.; An, Q.; Zhao, J.; Ma, J.; Sun, Z.; Chu, W. Adv. Synth. Catal. 2021, 363, 2806.
doi: 10.1002/adsc.202100168 |
|
| [16] |
Chen, R.; Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Org. Lett. 2018, 20, 3377.
doi: 10.1021/acs.orglett.8b01302 |
| [17] |
CCDC 2141840 contains the supplementary crystallographic data for this paper. These data are available free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif.
|
| [18] |
Zhao, D.; Luo, H.; Chen, B.; Chen, W.; Zhang, G.; Yu, Y. J. Org. Chem. 2018, 83, 7860.
doi: 10.1021/acs.joc.8b00734 |
| [19] |
Lozano, D.; Álvarez-Yebra, R.; López-Coll, R.; Lledó, A. Chem. Sci. 2019, 10, 10351.
doi: 10.1039/c9sc03158h pmid: 32110323 |
| [20] |
Example for the mechanism of SET, see: (a) Zhang, L. B.; Hao, X. Q.; Zhang, S. K.; Liu, Z. J.; Zheng, X. X.; Gong, J. F.; Niu, J. L.; Song, M. P. Angew. Chem., Int. Ed. 2015, 54, 272.
doi: 10.1002/anie.201409751 |
|
(b) Tan, G.; He, S.; Huang, X.; Liao, X.; Cheng, Y.; You, J. Angew. Chem., Int. Ed. 2016, 55, 10414.
doi: 10.1002/anie.201604580 |
|
|
(c) Guo, X. K.; Zhang, L. B.; Wei, D.; Niu, J. L. Chem. Sci. 2015, 6, 7059.
doi: 10.1039/C5SC01807B |
|
| [21] |
Xie, Y.; Yang, Y.; Huang, L.; Zhang, X.; Zhang, Y. Org. Lett. 2012, 14, 1238.
doi: 10.1021/ol300037p |
| [22] |
Wadhwa, K.; Yang, C.; West, P. R.; Deming, K. C.; Chemburkar, S. R.; Reddy, R. E. Synth. Commun. 2008, 38, 4434.
doi: 10.1080/00397910802369554 |
| [1] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [2] | 高师泉, 刘闯军, 杨俊锋, 张俊良. 钴催化的烯烃和炔烃的电化学还原偶联反应[J]. 有机化学, 2023, 43(4): 1559-1565. |
| [3] | 周鹏, 朱伟明, 张建涛, 肖朵朵, 郭祥峰, 刘卫兵. 钴催化芳基烯烃氧烷基化反应: 快速获得α-烷基取代苯乙酮衍生物[J]. 有机化学, 2023, 43(11): 3939-3944. |
| [4] | 顾海春, 靳新新, 李嘉琪, 李贺, 刘景林. 过渡金属催化N-芳基酞嗪的C—H键活化反应研究进展[J]. 有机化学, 2022, 42(9): 2682-2702. |
| [5] | 陈飞, 陶晟, 刘宁, 代斌. CNN型双核Cu(I)配合物室温催化固定CO2的直接羧基化反应[J]. 有机化学, 2022, 42(8): 2471-2480. |
| [6] | 付拯江, 曹晰晗, 尹健, 苟振宇, 伊学政, 蔡琥. 基于“一石二鸟”策略的羧基无痕导向其邻位C—H键官能团化反应[J]. 有机化学, 2022, 42(1): 67-74. |
| [7] | 罗欢欢, 裴娜, 张敬. 过渡金属催化氮原子导向的芳基邻位C—H键硼化反应研究进展[J]. 有机化学, 2021, 41(8): 2990-3001. |
| [8] | 王银银, 林晓婉, 张飘, 沈美华, 徐华栋, 徐德锋. 基于吡啶和1,3,5-三嗪的PNP钳状配体的设计合成以及在钴催化的末端炔烃半氢化反应中的应用[J]. 有机化学, 2021, 41(8): 3312-3320. |
| [9] | 孙名扬, 徐坤, 郭兵兵, 曾程初. 空气氧化的铜催化苯甲酸衍生物邻位C(sp2)—H键的硒化反应[J]. 有机化学, 2021, 41(6): 2302-2309. |
| [10] | 黄音君, 李金山, 李珅, 马军安. 钴催化2-芳基吲哚氧化去芳构化及与烯酰胺[3+2]环化反应[J]. 有机化学, 2021, 41(10): 4028-4038. |
| [11] | 孙越, 关瑞, 刘兆洪, 王也铭. 铁、钴、镍催化烯烃的硼氢化反应研究进展[J]. 有机化学, 2020, 40(4): 899-912. |
| [12] | 张梦帆, 李瑞鹏, 杨震, 冯若昆. 钴催化双齿导向基辅助的1-萘胺衍生物与醇的区域选择性碳氢键烷氧基化反应[J]. 有机化学, 2020, 40(3): 714-723. |
| [13] | 程彪, 陆鹏, 赵家金, 陆展. 钴催化芳香族烯烃的脱氢硅化反应[J]. 有机化学, 2019, 39(6): 1704-1710. |
| [14] | 孙义明, 丁奇峰, 于杨, 何益得, 黄菲. 钴催化C—H胺化反应的研究进展[J]. 有机化学, 2019, 39(12): 3363-3374. |
| [15] | 王亚琦, 尹强, 郭墩, 韩利民, 孙琪, 洪海龙, 索全伶. 超临界二氧化碳中羰基钴催化的端基炔烃环三聚反应研究[J]. 有机化学, 2019, 39(10): 2898-2905. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||