有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2229-2235.DOI: 10.6023/cjoc202202007 上一篇 下一篇
研究论文
李海琼, 尹梦云, 谢芬芬, 张正兵*( ), 韩盼*(
), 韩盼*( ), 敬林海*(
), 敬林海*( )
)
收稿日期:2022-02-06
修回日期:2022-03-11
发布日期:2022-08-09
通讯作者:
张正兵, 韩盼, 敬林海
作者简介:基金资助:
Haiqiong Li, Mengyun Yin, Fenfen Xie, Zhengbing Zhang( ), Pan Han(
), Pan Han( ), Linhai Jing(
), Linhai Jing( )
)
Received:2022-02-06
Revised:2022-03-11
Published:2022-08-09
Contact:
Zhengbing Zhang, Pan Han, Linhai Jing
About author:Supported by:文章分享
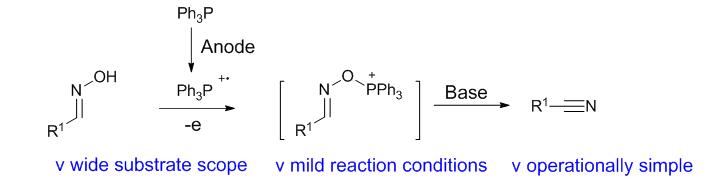
探索了一种通过电化学Appel反应合成腈的方法. 该方法具有操作简单、反应条件温和、环境友好的优点, 可以合成一系列芳香腈和脂肪腈类化合物. 在循环伏安实验和控制实验基础上, 提出了电化学Appel反应机理用于解释反应过程.
李海琼, 尹梦云, 谢芬芬, 张正兵, 韩盼, 敬林海. 通过电化学Appel反应合成腈[J]. 有机化学, 2022, 42(7): 2229-2235.
Haiqiong Li, Mengyun Yin, Fenfen Xie, Zhengbing Zhang, Pan Han, Linhai Jing. Synthesis of Nitrile via Electrochemical Appel Reaction[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2229-2235.
| Entry | Electrolyte | Solvent | Base | Yield b/% |
|---|---|---|---|---|
| 1 | nBu4NPF6 | CH3CN | NaHCO3 | 67 |
| 2c | nBu4NPF6 | CH3CN | NaHCO3 | 33 |
| 3d | nBu4NPF6 | CH3CN | NaHCO3 | 26 |
| 4e | nBu4NPF6 | CH3CN | NaHCO3 | 48 |
| 5 | nBu4NBF4 | CH3CN | NaHCO3 | 41 |
| 6 | Me4NBF4 | CH3CN | NaHCO3 | 55 |
| 7 | nBu4NClO4 | CH3CN | NaHCO3 | 60 |
| 8 | LiClO4 | CH3CN | NaHCO3 | 11 |
| 9 | TBAB | CH3CN | NaHCO3 | 85 (83)f |
| 10 | TBAI | CH3CN | NaHCO3 | 60 |
| 11 | TBAB | CH3CN | Na2CO3 | 70 |
| 12 | TBAB | CH3CN | K3PO4 | 46 |
| 13 | TBAB | CH3CN | Cs2CO3 | 69 |
| 14 | TBAB | CH3CN | Et3N | 44 |
| 15 | TBAB | THF | NaHCO3 | 0 |
| 16 | TBAB | DCM | NaHCO3 | 48 |
| 17 | TBAB | DMF | NaHCO3 | 15 |
| 18 | TBAB | Dioxane | NaHCO3 | 0 |
| 19g | TBAB | CH3CN | NaHCO3 | 64 |
| 20h | TBAB | CH3CN | NaHCO3 | 57 |
| 21i | TBAB | CH3CN | NaHCO3 | 50 |
| 22j | TBAB | CH3CN | NaHCO3 | 0 |
| Entry | Electrolyte | Solvent | Base | Yield b/% |
|---|---|---|---|---|
| 1 | nBu4NPF6 | CH3CN | NaHCO3 | 67 |
| 2c | nBu4NPF6 | CH3CN | NaHCO3 | 33 |
| 3d | nBu4NPF6 | CH3CN | NaHCO3 | 26 |
| 4e | nBu4NPF6 | CH3CN | NaHCO3 | 48 |
| 5 | nBu4NBF4 | CH3CN | NaHCO3 | 41 |
| 6 | Me4NBF4 | CH3CN | NaHCO3 | 55 |
| 7 | nBu4NClO4 | CH3CN | NaHCO3 | 60 |
| 8 | LiClO4 | CH3CN | NaHCO3 | 11 |
| 9 | TBAB | CH3CN | NaHCO3 | 85 (83)f |
| 10 | TBAI | CH3CN | NaHCO3 | 60 |
| 11 | TBAB | CH3CN | Na2CO3 | 70 |
| 12 | TBAB | CH3CN | K3PO4 | 46 |
| 13 | TBAB | CH3CN | Cs2CO3 | 69 |
| 14 | TBAB | CH3CN | Et3N | 44 |
| 15 | TBAB | THF | NaHCO3 | 0 |
| 16 | TBAB | DCM | NaHCO3 | 48 |
| 17 | TBAB | DMF | NaHCO3 | 15 |
| 18 | TBAB | Dioxane | NaHCO3 | 0 |
| 19g | TBAB | CH3CN | NaHCO3 | 64 |
| 20h | TBAB | CH3CN | NaHCO3 | 57 |
| 21i | TBAB | CH3CN | NaHCO3 | 50 |
| 22j | TBAB | CH3CN | NaHCO3 | 0 |
| [1] |
(a) Dworczak, R.; Fabian, W. M. F.; Biza, P.; Weikmann, M.; Junek, H. Dyes Pigm. 1995, 28, 297.
doi: 10.1016/0143-7208(95)00022-8 |
|
(b) Dworczak, R.; Fabian, W. M. F.; Pawar, B. N.; Junek, H. Dyes Pigm. 1995, 29, 65.
doi: 10.1016/0143-7208(95)00028-E |
|
|
(c) Pearce, E. M.; Weil, E. D.; Barinov, V. Y. Fire Smart Polymers (Fire and Polymers), American Chemical Society, 2001, pp. 37-48.
|
|
|
(d) Amr, M. A.; Mohamed, H. E.; Mohamed, S. E.; Hesham, R. E.-S.; Ismail, A. A. Curr. Org. Synth. 2018, 15, 487.
doi: 10.2174/1570179415666180403120140 |
|
| [2] |
Fatiadi, A. J. In Preparation and Synthetic Applications of Cyano Compounds, Eds.: Patai, S.; Rappaport, Z., Wiley, New York, 1983.
|
| [3] |
(a) Iyengar, B. S.; Dorr, R. T.; Remers, W. A. J. Med. Chem. 2004, 47, 218.
doi: 10.1021/jm030225v pmid: 17228871 |
|
(b) Romero, M.; Renard, P.; Caignard, D.-H.; Atassi, G.; Solans, X.; Constans, P.; Bailly, C.; Pujol, M. D. J. Med. Chem. 2007, 50, 294.
pmid: 17228871 |
|
| [4] |
Pascual, E.; Sivera, F.; Yasothan, U.; Kirkpatrick, P. Nat. Rev. Drug Discov. 2009, 8, 191.
doi: 10.1038/nrd2831 pmid: 19247302 |
| [5] |
Patat, A.; Paty, I.; Hindmarch, I. Hum. Psychopharmacol. 2001, 16, 369.
doi: 10.1002/hup.310 |
| [6] |
(a) Sica, D. A.; Prisant, L. M. J. Clin. Hypertens. (Shelton, CT, U. S.) 2007, 9, 1.
pmid: 19900016 |
|
(b) Cooper-DeHoff, R. M.; Handberg, E. M.; Mancia, G.; Zhou, Q.; Champion, A.; Legler, U. F.; Pepine, C. J. Expert Rev. Cardiovasc. Ther. 2009, 7, 1329.
doi: 10.1586/erc.09.102 pmid: 19900016 |
|
| [7] |
Noble, S.; McTavish, D. Drugs 1995, 50, 1032.
pmid: 8612470 |
| [8] |
(a) Shu, Z.; Ye, Y.; Deng, Y.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2013, 52, 10573.
doi: 10.1002/anie.201305731 |
|
(b) Liu, J.; Zheng, H.-X.; Yao, C.-Z.; Sun, B.-F.; Kang, Y.-B. J. Am. Chem. Soc. 2016, 138, 3294.
doi: 10.1021/jacs.6b00180 |
|
|
(c) Ge, J.-J.; Yao, C.-Z.; Wang, M.-M.; Zheng, H.-X.; Kang, Y.-B.; Li, Y. Org. Lett. 2016, 18, 228.
doi: 10.1021/acs.orglett.5b03367 |
|
|
(d) Yu, L.; Li, H.; Zhang, X.; Ye, J.; Liu, J.; Xu, Q.; Lautens, M. Org. Lett. 2014, 16, 1346.
doi: 10.1021/ol500075h |
|
|
(e) Zhuang, Y.-J.; Liu, J.; Kang, Y.-B. Tetrahedron Lett. 2016, 57, 5700.
doi: 10.1016/j.tetlet.2016.11.034 |
|
| [9] |
(a) Zhou, S.; Addis, D.; Das, S.; Junge, K.; Beller, M. Chem. Commun. 2009, 4883.
pmid: 29320204 |
|
(b) Shipilovskikh, S. A.; Vaganov, V. Y.; Denisova, E. I.; Rubtsov, A. E.; Malkov, A. V. Org. Lett. 2018, 20, 728.
doi: 10.1021/acs.orglett.7b03862 pmid: 29320204 |
|
| [10] |
(a) Zhou, W.; Zhang, L.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7094.
doi: 10.1002/anie.200903838 |
|
(b) Tseng, K.-N. T.; Rizzi, A. M.; Szymczak, N. K. J. Am. Chem. Soc. 2013, 135, 16352.
doi: 10.1021/ja409223a |
|
|
(c) Guo, S.; Wan, G.; Sun, S.; Jiang, Y.; Yu, J.-T.; Cheng, J. Chem. Commun. 2015, 51, 5085.
doi: 10.1039/C5CC01024A |
|
| [11] |
(a) Anderson, B. A.; Bell, E. C.; Ginah, F. O.; Harn, N. K.; Pagh, L. M.; Wepsiec, J. P. J. Org. Chem. 1998, 63, 8224.
doi: 10.1021/jo9808674 |
|
(b) Zanon, J.; Klapars, A.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125, 2890.
doi: 10.1021/ja0299708 |
|
|
(c) Cristau, H.-J.; Ouali, A.; Spindler, J.-F.; Taillefer, M. Chem.-Eur. J. 2005, 11, 2483.
doi: 10.1002/chem.200400979 |
|
|
(d) Pan, S.; Wu, F.; Yu, R.; Chen, W. J. Org. Chem. 2016, 81, 1558.
doi: 10.1021/acs.joc.5b02710 |
|
|
(e) Yan, G.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2017, 359, 4068.
doi: 10.1002/adsc.201700875 |
|
| [12] |
(a) Fang, C.; Li, M.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Jin, L.; Shen, Z. RSC Adv. 2017, 7, 1484.
doi: 10.1039/C6RA26435B pmid: 33795972 |
|
(b) Murugesan, K.; Senthamarai, T.; Sohail, M.; Sharif, M.; Kalevaru, N. V.; Jagadeesh, R. V. Green Chem. 2018, 20, 266.
doi: 10.1039/C7GC02627G pmid: 33795972 |
|
|
(c) Chen, H.; Sun, S.; Xi, H.; Hu, K.; Zhang, N.; Qu, J.; Zhou, Y. Tetrahedron Lett. 2019, 60, 1434.
doi: 10.1016/j.tetlet.2019.04.043 pmid: 33795972 |
|
|
(d) Zhan, W.; Tong, M.; Ji, L.; Zhang, H.; Ge, Z.; Wang, X.; Li, R.. Chin. Chem. Lett. 2019, 30, 973.
doi: 10.1016/j.cclet.2019.01.006 pmid: 33795972 |
|
|
(e) Mudshinge, S. R.; Potnis, C. S.; Xu, B.; Hammond, G. B. Green Chem. 2020, 22, 4161.
doi: 10.1039/d0gc00757a pmid: 33795972 |
|
|
(f) Hua, M.; Song, J.; Huang, X.; Liu, H.; Fan, H.; Wang, W.; He, Z.; Liu, Z.; Han, B. Angew. Chem., Int. Ed. 2021, 60, 21479.
doi: 10.1002/anie.202107996 pmid: 33795972 |
|
| [13] |
(a) Xu, J.-H.; Jiang, Q.; Guo, C.-C. J. Org. Chem. 2013, 78, 11881.
doi: 10.1021/jo401919h |
|
(b) Preger, Y.; Root, T. W.; Stahl, S. S. ACS Omega 2018, 3, 6091.
doi: 10.1021/acsomega.8b00911 |
|
|
(c) Vanoye, L.; Hammoud, A.; Gérard, H.; Barnes, A.; Philippe, R.; Fongarland, P.; de Bellefon, C.; Favre-Réguillon, A. ACS Catal. 2019, 9, 9705.
doi: 10.1021/acscatal.9b02779 |
|
|
(d) Murata, Y.; Iwasa, H.; Matsumura, M.; Yasuike, S. Chem. Pharm. Bull. 2020, 68, 679.
doi: 10.1248/cpb.c20-00228 |
|
|
(e) Takahashi, Y.; Tsuji, H.; Kawatsura, M. J. Org. Chem. 2020, 85, 2654.
doi: 10.1021/acs.joc.9b02705 |
|
|
(f) Lu, D.; Cui, J.; Yang, S.; Gong, Y. ACS Catal. 2021, 11, 4288.
doi: 10.1021/acscatal.1c00557 |
|
|
(g) Xiao, J.; Guo, F.; Li, Y.; Li, F.; Li, Q.; Tang, Z.-L. J. Org. Chem. 2021, 86, 2028.
doi: 10.1021/acs.joc.0c02794 |
|
| [14] |
(a) Li, Y.-T.; Liao, B.-S.; Chen, H.-P.; Liu, S.-T. Synthesis 2011, 2639.
pmid: 30240220 |
|
(b) Ma, X.-Y.; He, Y.; Lu, T.-T.; Lu, M. Tetrahedron 2013, 69, 2560.
doi: 10.1016/j.tet.2013.01.059 pmid: 30240220 |
|
|
(c) Ghosh, P.; Pariyar, G. C.; Saha, B.; Subba, R. Synth. Commun. 2016, 46, 685.
doi: 10.1080/00397911.2016.1167910 pmid: 30240220 |
|
|
(d) Hyodo, K.; Kitagawa, S.; Yamazaki, M.; Uchida, K. Chem. Asian J. 2016, 11, 1348.
doi: 10.1002/asia.201600085 pmid: 30240220 |
|
|
(e) Rapeyko, A.; Climent, M. J.; Corma, A.; Concepción, P.; Iborra, S. ACS Catal. 2016, 6, 4564.
doi: 10.1021/acscatal.6b00272 pmid: 30240220 |
|
|
(f) Sun, D.; Kitamura, E.; Yamada, Y.; Sato, S. Green Chem. 2016, 18, 3389.
doi: 10.1039/C6GC00384B pmid: 30240220 |
|
|
(g) Ding, R.; Liu, Y.; Han, M.; Jiao, W.; Li, J.; Tian, H.; Sun, B. J. Org. Chem. 2018, 83, 12939.
doi: 10.1021/acs.joc.8b02190 pmid: 30240220 |
|
|
(h) Zhang, D.; Huang, Y.; Zhang, E.; Yi, R.; Chen, C.; Yu, L.; Xu, Q. Adv. Synth. Catal. 2018, 360, 784.
doi: 10.1002/adsc.201701154 pmid: 30240220 |
|
|
(i) Ma, X.; Liu, D.; Chen, Z. Synth. Commun. 2021, 51, 3261.
doi: 10.1080/00397911.2021.1943752 pmid: 30240220 |
|
| [15] |
(a) Sperry, J. B.; Wright, D. L. Chem. Soc. Rev. 2006, 35, 605.
doi: 10.1039/b512308a pmid: 29498518 |
|
(b) Jutand, A. Chem. Rev. 2008, 108, 2300.
doi: 10.1021/cr068072h pmid: 29498518 |
|
|
(c) Yoshida, J.-I.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265.
doi: 10.1021/cr0680843 pmid: 29498518 |
|
|
(d) Francke, R.; Little, R. D. Chem. Soc. Rev. 2014, 43, 2492.
doi: 10.1039/c3cs60464k pmid: 29498518 |
|
|
(e) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230.
doi: 10.1021/acs.chemrev.7b00397 pmid: 29498518 |
|
|
(f) Ma, C.; Fang, P.; Mei, T.-S. ACS Catal. 2018, 8, 7179.
doi: 10.1021/acscatal.8b01697 pmid: 29498518 |
|
|
(g) Moeller, K. D. Chem. Rev. 2018, 118, 4817.
doi: 10.1021/acs.chemrev.7b00656 pmid: 29498518 |
|
|
(h) Yoshida, J.-I.; Shimizu, A.; Hayashi, R. Chem. Rev. 2018, 118, 4702.
doi: 10.1021/acs.chemrev.7b00475 pmid: 29498518 |
|
|
(i) Marken, F.; Wadhawan, J. D. Acc. Chem. Res. 2019, 52, 3325.
doi: 10.1021/acs.accounts.9b00480 pmid: 29498518 |
|
|
(j) Xiong, P.; Xu, H.-C. Acc. Chem. Res. 2019, 52, 3339.
doi: 10.1021/acs.accounts.9b00472 pmid: 29498518 |
|
|
(k) Yuan, Y.; Lei, A. Acc. Chem. Res. 2019, 52, 3309.
doi: 10.1021/acs.accounts.9b00512 pmid: 29498518 |
|
|
(l) Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Acc. Chem. Res. 2020, 53, 300.
doi: 10.1021/acs.accounts.9b00603 pmid: 29498518 |
|
|
(m) Siu, J. C.; Fu, N.; Lin, S. Acc. Chem. Res. 2020, 53, 547.
doi: 10.1021/acs.accounts.9b00529 pmid: 29498518 |
|
| [16] |
(a) Mo, Z.-Y.; Swaroop, T. R.; Tong, W.; Zhang, Y.-Z.; Tang, H.-T.; Pan, Y.-M.; Sun, H.-B.; Chen, Z.-F. Green Chem. 2018, 20, 4428.
doi: 10.1039/C8GC02143K |
|
(b) Zhang, Y.-Z.; Mo, Z.-Y.; Wang, H.-S.; Wen, X.-A.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 3807.
doi: 10.1039/C9GC01201J |
|
|
(c) Wang, X.-Y.; Zhong, Y.-F.; Mo, Z.-Y.; Wu, S.-H.; Xu, Y.-L.; Tang, H.-T.; Pan, Y.-M. Adv. Synth. Catal. 2021, 363, 208.
doi: 10.1002/adsc.202001192 |
|
|
(d) Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233.
|
|
|
(e) Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Green Synth. Catal. 2021, 2, 19.
|
|
|
(f) Zhang, S.; Ye, X.; Wojtas, L.; Hao, W.; Shi, X. Green Synth. Catal. 2021, 2, 82.
|
|
| [17] |
Libendi, S. S.; Demizu, Y.; Onomura, O. Org. Biomol. Chem. 2009, 7, 351.
doi: 10.1039/B816598J |
| [18] |
(a) Cui, T.; Zhan, Y.; Dai, C.; Lin, J.; Liu, P.; Sun, P. J. Org. Chem. 2021, 86, 15897.
doi: 10.1021/acs.joc.0c03026 |
|
(b) Gao, J.; Weng, X.; Ma, C.; Xu, X.; Fang, P.; Mei, T. Chin. J. Org. Chem. 2021, 41, 3223. (in Chinese)
doi: 10.6023/cjoc202103049 |
|
|
( 高君青, 翁信军, 马聪, 徐学涛, 方萍, 梅天胜, 有机化学, 2021, 41, 3223.)
doi: 10.6023/cjoc202103049 |
|
| [19] |
Dai, J.-J.; Huang, Y.-B.; Fang, C.; Guo, Q.-X.; Fu, Y. ChemSusChem 2012, 5, 617.
doi: 10.1002/cssc.201100776 |
| [20] |
Ye, J.-Q.; Zhang, Z.-L.; Zha, Z.-G.; Wang, Z.-Y. Chin. Chem. Lett. 2014, 25, 1112.
doi: 10.1016/j.cclet.2014.04.024 |
| [21] |
Qu, Q.; Gao, X.; Gao, J.; Yuan, G. Sci. China Chem. 2015, 58, 747.
doi: 10.1007/s11426-015-5331-z |
| [22] |
Shono, T.; Matsumura, Y.; Tsubata, K.; Kamada, T.; Kishi, K.. J. Org. Chem. 1989, 54, 2249.
doi: 10.1021/jo00270a044 |
| [23] |
Hartmer, M. F.; Waldvogel, S. R. Chem. Commun. 2015, 51, 16346.
doi: 10.1039/C5CC06437F |
| [24] |
(a) Ohmori, H.; Nakai, S.; Sekiguchi, M.; Masui, M. Chem. Pharm. Bull. 1980, 28, 910.
doi: 10.1248/cpb.28.910 |
|
(b) Xu, Z.; Zheng, Y.; Wang, Z.; Shao, X.; Tian, L.; Wang, Y. Chem. Commun. 2019, 55, 15089.
doi: 10.1039/C9CC08622F |
|
| [25] |
(a) de Andrade, V. S. C.; de Mattos, M. C. S. Curr. Org. Synth. 2015, 12, 309.
doi: 10.2174/1570179412666150305231358 |
|
(b) Li, Z.; Sun, W.; Wang, X.; Li, L.; Zhang, Y.; Li, C. J. Am. Chem. Soc. 2021, 143, 3536.
doi: 10.1021/jacs.0c13093 |
|
| [26] |
(a) Ban, Y.-L.; Dai, J.-L.; Jin, X.-L.; Zhang, Q.-B.; Liu, Q. Chem. Commun. 2019, 55, 9701.
doi: 10.1039/C9CC05354A |
|
(b) Schäfer, R. J. B.; Monaco, M. R.; Li, M.; Tirla, A.; Rivera- Fuentes, P.; Wennemers, H. J. Am. Chem. Soc. 2019, 141, 18644.
doi: 10.1021/jacs.9b07632 |
| [1] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [2] | 张俊颖, 赵晓静, 李干鹏, 何永辉. 室温下电化学合成保护型有机硼酸RB(dan)[J]. 有机化学, 2023, 43(5): 1815-1823. |
| [3] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [4] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [5] | 付拯江, 杨振江, 孙丽, 尹健, 伊学政, 蔡琥, 雷爱文. 无金属条件下亚磺酸钠与酚类化合物形成芳基磺酸酯的电化学合成反应[J]. 有机化学, 2022, 42(2): 600-606. |
| [6] | 锅小龙, 王玉贤, 赵志强, 王庆, 左剑, 王陆瑶. 电化学氧化下喹喔啉-2(1H)-酮的三氟甲基化及电描述符对反应性能的评价[J]. 有机化学, 2022, 42(2): 641-649. |
| [7] | 李红霞, 陈棚, 伍智林, 陆雨函, 彭俊梅, 陈锦杨, 何卫民. 电化学促进的五元芳香杂环与硫氰酸铵氧化交叉脱氢偶联反应[J]. 有机化学, 2022, 42(10): 3398-3404. |
| [8] | 赵志恒, 李鸣, 周娅琴, 何永辉, 张丽珠, 李干鹏, 谷利军. 电化学脱氢[3+2]环化反应合成取代的1,2,4-三氮唑衍生物[J]. 有机化学, 2021, 41(6): 2476-2484. |
| [9] | 周娅琴, 赵志恒, 曾亮, 李鸣, 何永辉, 谷利军. 卤素盐参与下有机电合成含氮杂环化合物的研究进展[J]. 有机化学, 2021, 41(3): 1072-1080. |
| [10] | 李文艺, 唐胤恒, 欧阳文韬, 陆雨函, 陈锦杨, 何卫民. N-无保护苯胺电化学硒醚化反应构建4-(烃基硒代)苯胺[J]. 有机化学, 2021, 41(12): 4766-4772. |
| [11] | 吴红谕, 于贤勇, 曹忠. α-酮酸、异腈和水的电化学三组分合成α-酮酰胺[J]. 有机化学, 2021, 41(12): 4712-4717. |
| [12] | 徐鹤华, 孟祥太, 郑煜, 罗金岳, 黄申林. 邻炔基苯胺的电化学环化合成3-碘吲哚[J]. 有机化学, 2021, 41(12): 4696-4703. |
| [13] | 蔡卫, 黄有. 有机膦氧化还原催化反应研究进展[J]. 有机化学, 2021, 41(10): 3903-3913. |
| [14] | 李梦帆, 王榕, 郝文娟, 姜波. 电催化合成2,5-二取代1,3,4-噁二唑衍生物[J]. 有机化学, 2020, 40(6): 1540-1548. |
| [15] | 王向阳, 徐学涛, 王振华, 方萍, 梅天胜. 过渡金属催化的不对称电化学进展[J]. 有机化学, 2020, 40(11): 3738-3747. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||