有机化学 ›› 2022, Vol. 42 ›› Issue (9): 2774-2792.DOI: 10.6023/cjoc202203008 上一篇 下一篇
综述与进展
收稿日期:2022-03-02
修回日期:2022-05-07
发布日期:2022-05-31
通讯作者:
赵军锋
基金资助:
Changliu Wanga, Yongli Zhaoa, Junfeng Zhaob( )
)
Received:2022-03-02
Revised:2022-05-07
Published:2022-05-31
Contact:
Junfeng Zhao
Supported by:文章分享

蛋白质是生物体内含量最丰富的生物大分子, 参与了几乎所有生命活动的进程. 蛋白质的化学修饰是研究与调控其理化性质和生物功能的有效方式, 当前大部分蛋白质的化学修饰是基于氨基酸残基侧链上活泼官能团的反应性来实现. 在20种天然氨基酸中, 半胱氨酸由于其巯基具有独特的亲核性、较低的氧化还原电势以及较低的自然丰度等特点, 成为了科学家研究蛋白质化学修饰的首选. 总结了近些年具有代表性的基于半胱氨酸的蛋白质化学修饰的研究进展, 阐述了相关研究的难点, 并对其未来发展方向进行了展望.
王长流, 赵永丽, 赵军锋. 基于半胱氨酸的蛋白质化学修饰研究进展[J]. 有机化学, 2022, 42(9): 2774-2792.
Changliu Wang, Yongli Zhao, Junfeng Zhao. Recent Advances in Chemical Protein Modification via Cysteine[J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2774-2792.

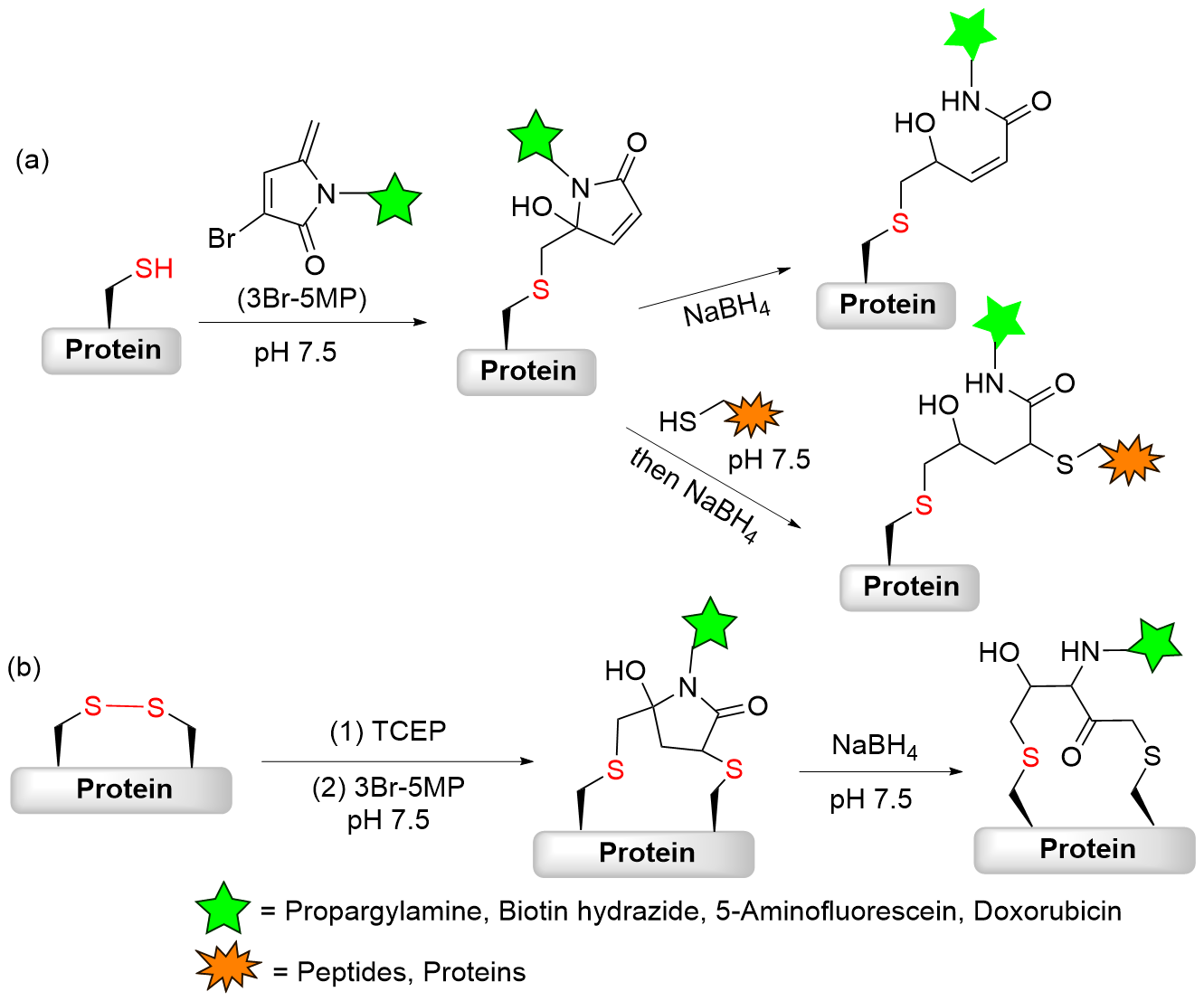
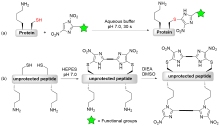
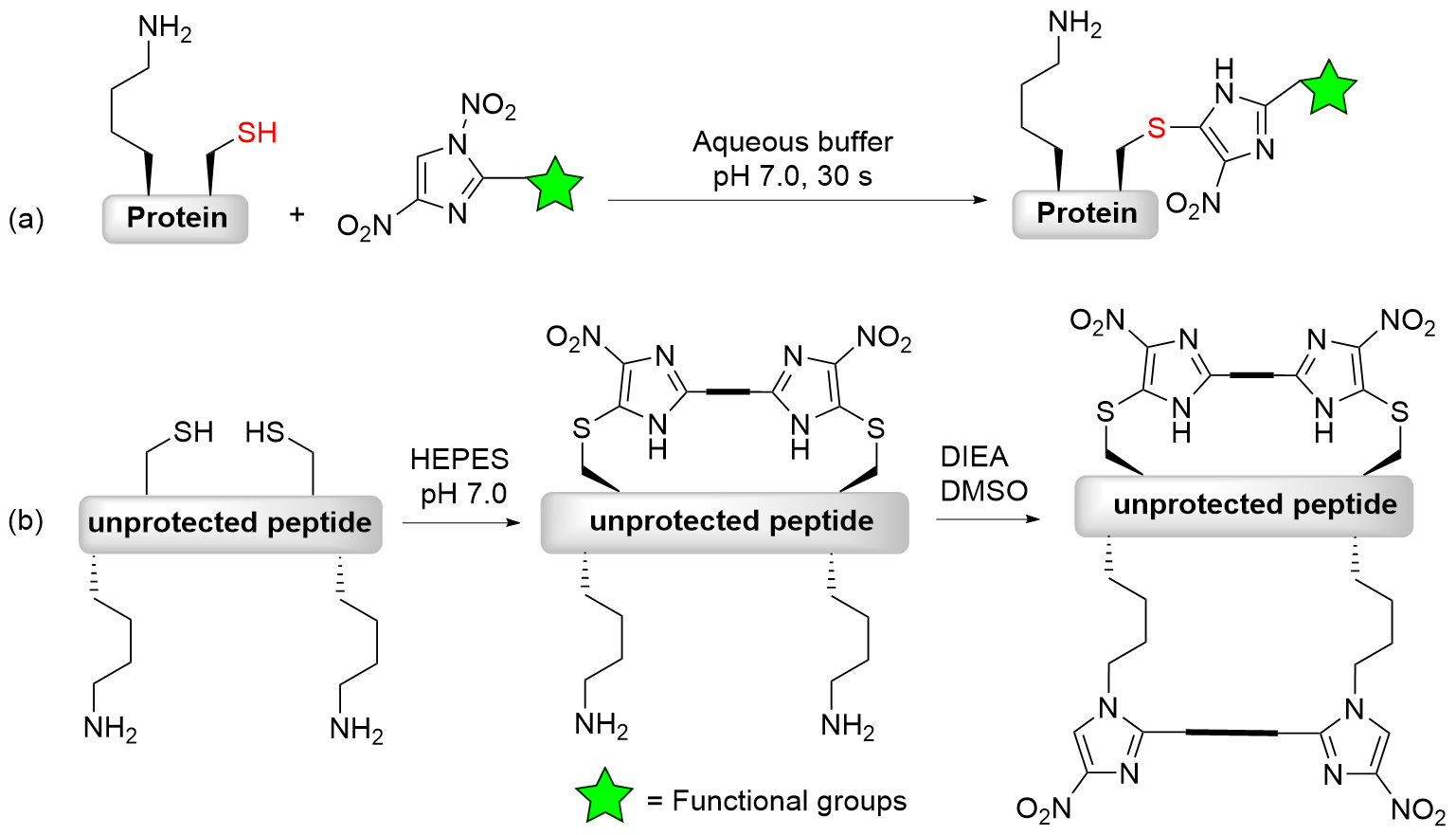
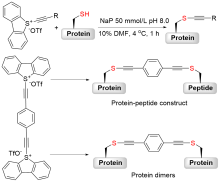
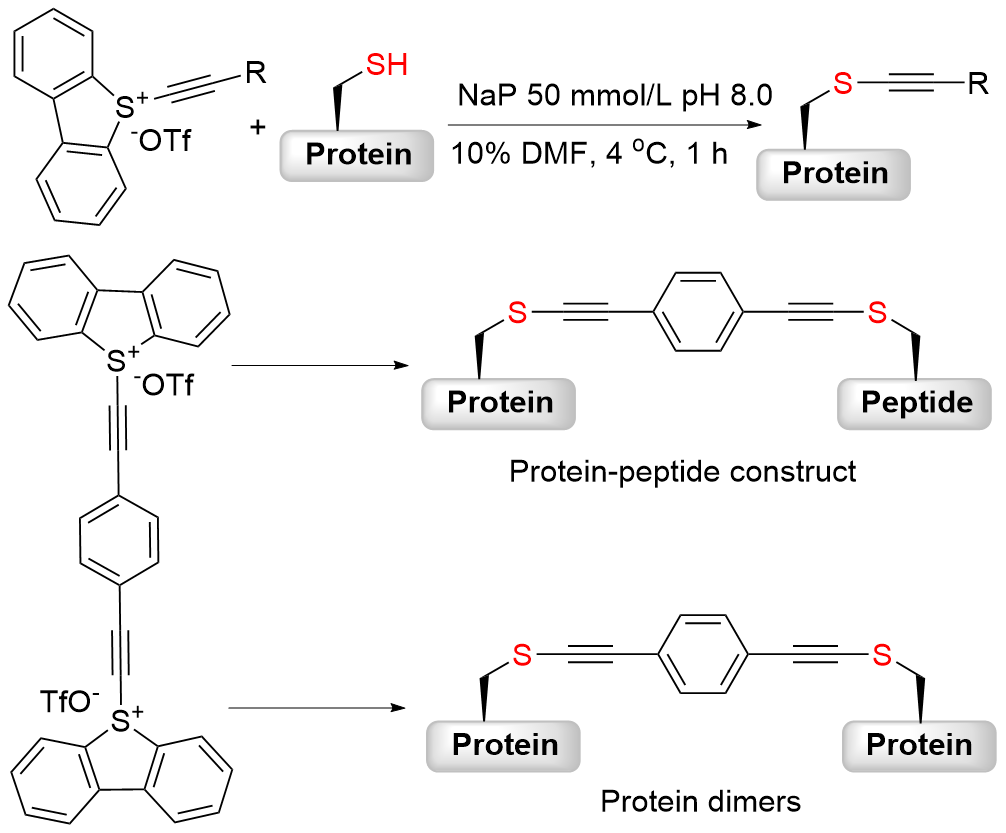
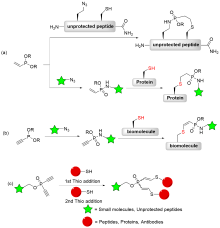
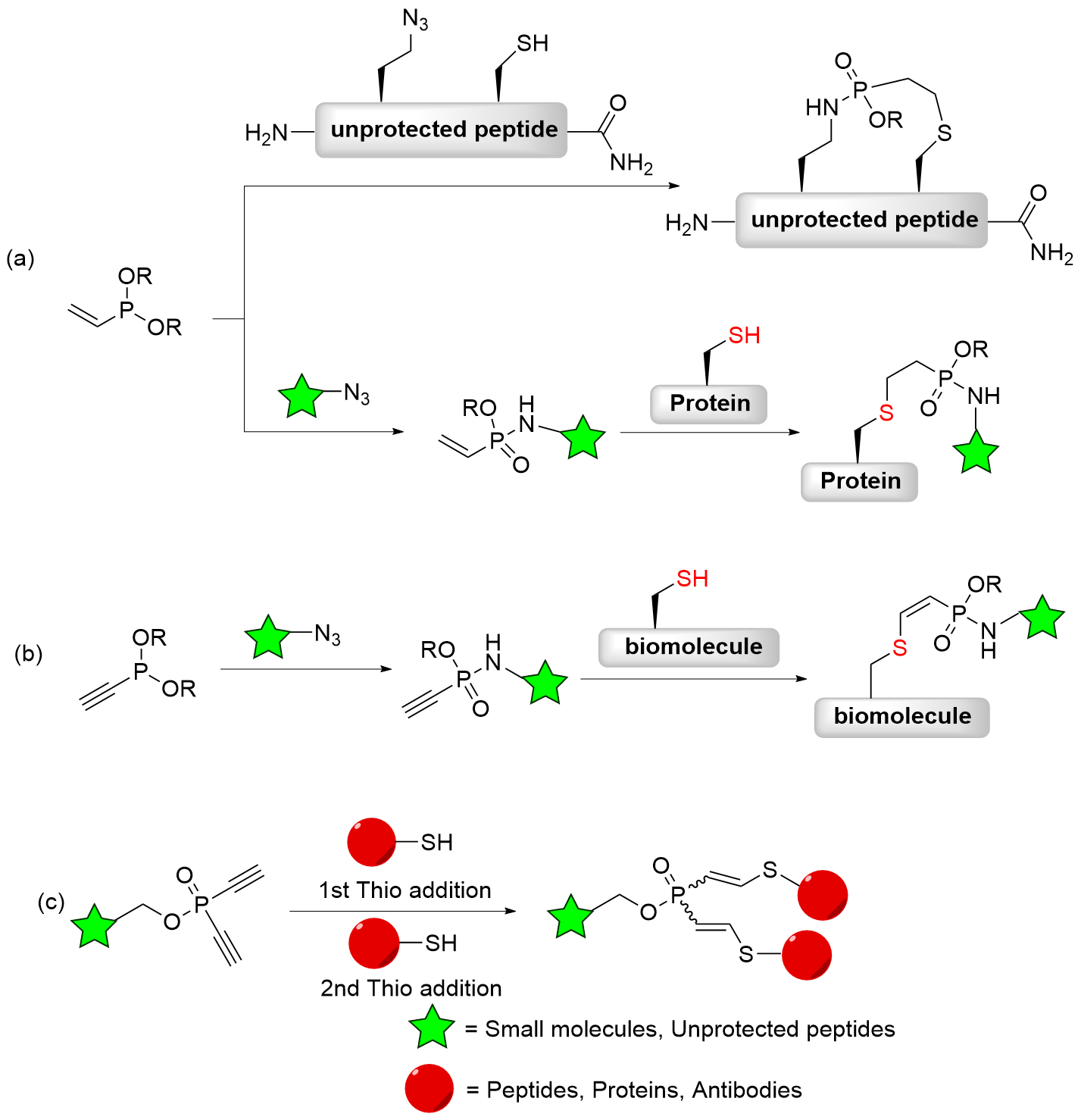
| [1] |
Walsh, C. T.; Garneau-Tsodikova, S.; Gatto, G. J. Jr. Angew. Chem. Int. Ed. 2005, 44, 7342.
doi: 10.1002/anie.200501023 |
| [2] |
Chatterjee, S.; Moon, S.; Hentschel, F.; Gilmore, K.; Seeberger, P. H. J. Am. Chem. Soc. 2018, 140, 11942.
doi: 10.1021/jacs.8b04525 pmid: 30125122 |
| [3] |
Bucci, M. Nat. Chem. Biol. 2018, 14, 525.
|
| [4] |
Diallo, I.; Seve, M.; Cunin, V.; Minassian, F.; Poisson, J.-F.; Michelland, S.; Bourgoin-Voillard, S. Expert Rev. Proteomics 2019, 16, 139.
doi: 10.1080/14789450.2019.1559061 |
| [5] |
Doerr, A. Nat. Med. 2018, 15, 651.
|
| [6] |
Prescher, J. A.; Bertozzi, C. R. Nat. Chem. Biol. 2005, 1, 13.
doi: 10.1038/nchembio0605-13 |
| [7] |
Kennedy, P. J.; Oliveira, C.; Granja, P. L. Pharmacol. Ther. 2017, 177, 129.
doi: 10.1016/j.pharmthera.2017.03.004 |
| [8] |
Lawrence, P. B.; Price, J. L. Curr. Opin. Chem. Biol. 2016, 34, 88.
doi: S1367-5931(16)30103-X pmid: 27580482 |
| [9] |
Schlatzer, T.; Kriegesmann, J.; Schroder, H.; Trobe, M.; Lembacher-Fadum, C.; Santner, S.; Kravchuk, A. V.; Becker, C. F. W.; Breinbauer, R. J. Am. Chem. Soc. 2019, 141, 14931.
doi: 10.1021/jacs.9b08279 pmid: 31469558 |
| [10] |
(a) Yang, M.-Y.; Chen, P. Acta Chim. Sinica 2015, 73, 783. (in Chinese)
pmid: 31657212 |
|
(杨麦云, 陈鹏, 化学学报, 2015, 73, 783.)
doi: 10.6023/A15030214 pmid: 31657212 |
|
|
(b) Zheng, Y.; Zhai, L.; Zhao, Y.; Wu, C. J. Am. Chem. Soc. 2015, 137, 15094.
doi: 10.1021/jacs.5b10779 pmid: 31657212 |
|
|
(c) Luo, Q.; Tao, Y.; Sheng, W.; Lu, J.; Wang, H. Nat. Commun. 2019, 10, 142.
doi: 10.1038/s41467-018-08010-2 pmid: 31657212 |
|
|
(d) Zhang, Y.; Zhang, Q.; Wong, C. T. T.; Li, X. J. Am. Chem. Soc. 2019, 141, 12274.
doi: 10.1021/jacs.9b03623 pmid: 31657212 |
|
|
(e) Zhang, Q.; Zhang, Y.; Liu, H.; Chow, H. Y.; Tian, R.; Eva Fung, Y. M.; Li, X. Biochemistry 2020, 59, 175.
doi: 10.1021/acs.biochem.9b00787 pmid: 31657212 |
|
|
(f) Zhao, K.; Lim, Y.-J.; Liu, Z.; Long, H.; Sun, Y.; Hu, J.-J.; Zhao, C.; Tao, Y.; Zhang, X.; Li, D.; Li, Y.-M.; Liu, C. Proc. Natl. Acad. Sci. 2020, 117, 20305.
doi: 10.1073/pnas.1922741117 pmid: 31657212 |
|
|
(g) Wang, S.; Zhou, Q.; Chen, X.; Luo, R.-H.; Li, Y.; Liu, X.; Yang, L.-M.; Zheng, Y.-T.; Wang, P. Nat. Commun. 2021, 12, 2257.
doi: 10.1038/s41467-021-22654-7 pmid: 31657212 |
|
| [11] |
Chambers, I.; Frampton, J.; Goldfarb, P.; Affara, N.; Mcbain, W.; Harrison, P. R. EMBO J. 1986, 5, 1221.
doi: 10.1002/j.1460-2075.1986.tb04350.x pmid: 3015592 |
| [12] |
Giles, N. M.; Watts, A. B.; Giles, G. I.; Fry, F. H.; Littlechild, J. A.; Jacob, C. Chem. Biol. 2003, 10, 677.
doi: 10.1016/S1074-5521(03)00174-1 |
| [13] |
Marino, S. M.; Gladyshev, V. N. J. Mol. Biol. 2010, 404, 902.
doi: 10.1016/j.jmb.2010.09.027 |
| [14] |
(a) Chalker, J. M.; Bernardes, G. J.; Lin, Y. A.; Davis, B. G. Chem.- Asian J. 2009, 4, 630.
doi: 10.1002/asia.200800427 pmid: 26789551 |
|
(b) Gunnoo, S. B.; Madder, A. ChemBioChem 2016, 17, 529.
doi: 10.1002/cbic.201500667 pmid: 26789551 |
|
|
(c) Ochtrop, P.; Hackenberger, C. P. R. Curr. Opin. Chem. Biol. 2020, 58, 28.
doi: 10.1016/j.cbpa.2020.04.017 pmid: 26789551 |
|
| [15] |
Goddard, D. R., Michaelis, L. J. Biol. Chem. 1935, 112, 361.
doi: 10.1016/S0021-9258(18)74993-4 |
| [16] |
Nielsen, M. L.; Vermeulen, M.; Bonaldi, T.; Cox, J.; Moroder, L.; Mann, M. Nat. Med. 2008, 5, 459.
doi: 10.1038/7458 |
| [17] |
Zhang, C.; Vinogradova, E. V.; Spokoyny, A. M.; Buchwald, S. L.; Pentelute, B. L. Angew. Chem., Int. Ed. 2019, 58, 4810.
doi: 10.1002/anie.201806009 pmid: 30399206 |
| [18] |
Chalker, J. M.; Wood, C. S. C.; Davis, B. G. J. Am. Chem. Soc. 2009, 131, 16346.
doi: 10.1021/ja907150m pmid: 19852502 |
| [19] |
Spokoyny, A. M.; Zou, Y.; Ling, J. J.; Yu, H.; Lin, Y. S.; Pentelute, B. L. J. Am. Chem. Soc. 2013, 135, 5946.
doi: 10.1021/ja400119t |
| [20] |
Zhang, C.; Spokoyny, A. M.; Zou, Y.; Simon, M. D.; Pentelute, B. L. Angew. Chem., Int. Ed. 2013, 52, 14001.
doi: 10.1002/anie.201306430 pmid: 24222025 |
| [21] |
Zhang, C.; Welborn, M.; Zhu, T.; Yang, N. J.; Santos, M. S.; Van Voorhis, T.; Pentelute, B. L. Nat. Chem. 2015, 8, 120.
doi: 10.1038/nchem.2413 |
| [22] |
Embaby, A. M.; Schoffelen, S.; Kofoed, C.; Meldal, M.; Diness, F. Angew. Chem., Int. Ed. 2018, 57, 8022.
doi: 10.1002/anie.201712589 pmid: 29469231 |
| [23] |
Bell, O.; Tiwari, V. K.; Thoma, N. H.; Schubeler, D. Nat. Rev. Genet. 2011, 12, 554.
|
| [24] |
Chu, G.-C.; Pan, M.; Li, J.; Liu, S.; Zuo, C.; Tong, Z.-B.; Bai, J.-S.; Gong, Q.; Ai, H.; Fan, J.; Meng, X.; Huang, Y.-C.; Shi, J.; Deng, H.; Tian, C.; Li, Y.-M.; Liu, L. J. Am. Chem. Soc. 2019, 141, 3654.
doi: 10.1021/jacs.8b13213 |
| [25] |
Diamantis, N.; Banerji, U. Br. J. Cancer 2016, 114, 362.
doi: 10.1038/bjc.2015.435 |
| [26] |
Walsh, S. J.; Bargh, J. D.; Dannheim, F. M.; Hanby, A. R.; Seki, H.; Counsell, A. J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; Isidro-Llobet, A.; Parker, J. S.; Carroll, J. S.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 1305.
doi: 10.1039/D0CS00310G |
| [27] |
(a) Tedaldi, L. M.; Smith, M. E.; Nathani, R. I.; Baker, J. R. Chem. Commun. 2009, 6583.
pmid: 34760149 |
|
(b) Kang, M. S.; Kong, T. W. S.; Khoo, J. Y. X.; Loh, T.-P. Chem. Sci. 2021, 12, 13613.
doi: 10.1039/d1sc02973h pmid: 34760149 |
|
| [28] |
Christie, R. J.; Fleming, R.; Bezabeh, B.; Woods, R.; Mao, S.; Harper, J.; Joseph, A.; Wang, Q.; Xu, Z.-Q.; Wu, H.; Gao, C.; Dimasi, N. J. Controlled Release 2015, 220, 660.
doi: 10.1016/j.jconrel.2015.09.032 |
| [29] |
Lyon, R. P.; Setter, J. R.; Bovee, T. D.; Doronina, S. O.; Hunter, J. H.; Anderson, M. E.; Balasubramanian, C. L.; Duniho, S. M.; Leiske, C. I.; Li, F.; Senter, P. D. Nat. Biotechnol. 2014, 32, 1059.
doi: 10.1038/nbt.2968 |
| [30] |
Ravasco, J.; Faustino, H.; Trindade, A.; Gois, P. M. P. Chem.-Eur. J. 2019, 25, 43.
doi: 10.1002/chem.201803174 pmid: 30095185 |
| [31] |
Huang, W.; Wu, X.; Gao, X.; Yu, Y.; Lei, H.; Zhu, Z.; Shi, Y.; Chen, Y.; Qin, M.; Wang, W.; Cao, Y. Nat. Chem. 2019, 11, 310.
doi: 10.1038/s41557-018-0209-2 |
| [32] |
Bernardim, B.; Cal, P. M.; Matos, M. J.; Oliveira, B. L.; Martinez-Saez, N.; Albuquerque, I. S.; Perkins, E.; Corzana, F.; Burtoloso, A. C.; Jimenez-Oses, G.; Bernardes, G. J. Nat. Commun. 2016, 7, 13128.
doi: 10.1038/ncomms13128 pmid: 27782215 |
| [33] |
Ariyasu, S.; Hayashi, H.; Xing, B.; Chiba, S. Bioconjugate Chem. 2017, 28, 897.
doi: 10.1021/acs.bioconjchem.7b00024 pmid: 28212596 |
| [34] |
Zhang, Y.; Zhou, X.; Xie, Y.; Greenberg, M. M.; Xi, Z.; Zhou, C. J. Am. Chem. Soc. 2017, 139, 6146.
doi: 10.1021/jacs.7b00670 |
| [35] |
Zhang, Y.; Zang, C.; An, G.; Shang, M.; Cui, Z.; Chen, G.; Xi, Z.; Zhou, C. Nat. Commun. 2020, 11, 1015.
doi: 10.1038/s41467-020-14757-4 |
| [36] |
Yu, J.; Yang, X.; Sun, Y.; Yin, Z. Angew. Chem., Int. Ed. 2018, 57, 11598.
doi: 10.1002/anie.201804801 |
| [37] |
Huang, R.; Li, Z.; Sheng, Y.; Yu, J.; Wu, Y.; Zhan, Y.; Chen, H.; Jiang, B. Org. Lett. 2018, 20, 6526.
doi: 10.1021/acs.orglett.8b02849 pmid: 30284842 |
| [38] |
Hoogenboom, R. Angew. Chem., Int. Ed. 2010, 49, 3415.
doi: 10.1002/anie.201000401 pmid: 20394091 |
| [39] |
(a) Frei, R.; Waser, J. J. Am. Chem. Soc. 2013, 135, 9620.
doi: 10.1021/ja4044196 |
|
(b) Frei, R.; Wodrich, M. D.; Hari, D. P.; Borin, P.-A.; Chauvier, C.; Waser, J. J. Am. Chem. Soc. 2014, 136, 16563.
doi: 10.1021/ja5083014 |
|
| [40] |
Abegg, D.; Frei, R.; Cerato, L.; Prasad Hari, D.; Wang, C.; Waser, J.; Adibekian, A. Angew. Chem., Int. Ed. 2015, 54, 10852.
doi: 10.1002/anie.201505641 |
| [41] |
Tessier, R.; Ceballos, J.; Guidotti, N.; Simonet-Davin, R.; Fierz, B.; Waser, J. Chem 2019, 5, 2243.
doi: 10.1016/j.chempr.2019.06.002 |
| [42] |
Tessier, R.; Nandi, R. K.; Dwyer, B. G.; Abegg, D.; Sornay, C.; Ceballos, J.; Erb, S.; Cianferani, S.; Wagner, A.; Chaubet, G.; Adibekian, A.; Waser, J. Angew. Chem., Int. Ed. 2020, 59, 10961.
doi: 10.1002/anie.202002626 pmid: 32233093 |
| [43] |
(a) Ceballos, J.; Grinhagena, E.; Sangouard, G.; Heinis, C.; Waser, J. Angew. Chem., Int. Ed. 2021, 60, 9022.
doi: 10.1002/anie.202014511 pmid: 33450121 |
|
(b) Ceballos, J.; Grinhagena, E.; Sangouard, G.; Heinis, C.; Waser, J. Angew. Chem., Int. Ed. 2021, 60, 9022.
doi: 10.1002/anie.202014511 pmid: 33450121 |
|
|
(c) Allouche, E. M. D.; Grinhagena, E.; Waser, J. Angew. Chem., Int. Ed. 2021, 60, 2.
doi: 10.1002/anie.202014556 pmid: 33450121 |
|
| [44] |
Zhang, C.; Dai, P.; Vinogradov, A. A.; Gates, Z. P.; Pentelute, B. L. Angew. Chem., Int. Ed. 2018, 57, 6459.
doi: 10.1002/anie.201800860 pmid: 29575377 |
| [45] |
Laserna, V.; Istrate, A.; Kafuta, K.; Hakala, T. A.; Knowles, T. P. J.; Alcarazo, M.; Bernardes, G. J. L. Bioconjugate Chem. 2021, 32, 1570.
doi: 10.1021/acs.bioconjchem.1c00317 pmid: 34232618 |
| [46] |
Truce, W. E.; Tichenor, G. J. W. J. Org. Chem. 1972, 37, 2391.
doi: 10.1021/jo00980a007 |
| [47] |
Arjona, O.; Iradier, F.; Medel, R.; Plumet, J. J. Org. Chem. 1999, 64, 6090.
doi: 10.1021/jo990308c |
| [48] |
Arjona, O.; Medel, R. o.; Rojas, J.; Costa, A. M.; Vilarrasa, J. Tetrahedron Lett. 2003, 44, 6369.
doi: 10.1016/S0040-4039(03)01614-9 |
| [49] |
Shiu, H. Y.; Chan, T. C.; Ho, C. M.; Liu, Y.; Wong, M. K.; Che, C. M. Chem.-Eur. J. 2009, 15, 3839.
doi: 10.1002/chem.200800669 |
| [50] |
Matos, M. J.; Navo, C. D.; Hakala, T.; Ferhati, X.; Guerreiro, A.; Hartmann, D.; Bernardim, B.; Saar, K. L.; Companon, I.; Corzana, F.; Knowles, T. P. J.; Jimenez-Oses, G.; Bernardes, G. J. L. Angew. Chem., Int. Ed. 2019, 58, 6640.
doi: 10.1002/anie.201901405 |
| [51] |
Vallee, M. R.; Majkut, P.; Wilkening, I.; Weise, C.; Muller, G.; Hackenberger, C. P. Org. Lett. 2011, 13, 5440.
doi: 10.1021/ol2020175 |
| [52] |
(a) Kasper, M. A.; Glanz, M.; Stengl, A.; Penkert, M.; Klenk, S.; Sauer, T.; Schumacher, D.; Helma, J.; Krause, E.; Cardoso, M. C.; Leonhardt, H.; Hackenberger, C. P. R. Angew. Chem., Int. Ed. 2019, 58, 11625.
doi: 10.1002/anie.201814715 |
|
(b) Park, Y.; Baumann, A. L.; Moon, H.; Byrne, S.; Kasper, M. A.; Hwang, S.; Sun, H.; Baik, M. H.; Hackenberger, C. P. R. Chem. Sci. 2021, 12, 8141.
doi: 10.1039/D1SC01730F |
|
| [53] |
Kasper, M.-A.; Glanz, M.; Oder, A.; Schmieder, P.; von Kries, J. P.; Hackenberger, C. P. R. Chem. Sci. 2019, 10, 6322.
doi: 10.1039/C9SC01345H |
| [54] |
Kasper, M. A.; Stengl, A.; Ochtrop, P.; Gerlach, M.; Stoschek, T.; Schumacher, D.; Helma, J.; Penkert, M.; Krause, E.; Leonhardt, H.; Hackenberger, C. P. R. Angew. Chem., Int. Ed. 2019, 58, 11631.
doi: 10.1002/anie.201904193 |
| [55] |
Stieger, C. E.; Franz, L.; Korlin, F.; Hackenberger, C. P. R. Angew. Chem., Int. Ed. 2021, 60, 1.
|
| [56] |
Abbas, A.; Xing, B.; Loh, T.-P. Angew. Chem., Int. Ed. 2014, 53, 7491.
doi: 10.1002/anie.201403121 pmid: 24889524 |
| [57] |
(a) Peng, Z.; Zhang, Z.; Tu, Y.; Zeng, X.; Zhao, J. Org. Lett. 2018, 20, 5688.
doi: 10.1021/acs.orglett.8b02409 |
|
(b) Peng, Z.; Zhang, Z.; Zeng, X.; Tu, Y.; Zhao, J. Adv. Synth. Catal. 2019, 361, 4489.
doi: 10.1002/adsc.201900734 |
|
| [58] |
Wang, C.; Zhao, Z.; Ghadir, R.; Li, Y.; Zhao, Y.; Metanis, N.; Zhao, J. ChemRxiv 2021, doi: 10.26434/chemrxiv-2021-9gxpp.
doi: 10.26434/chemrxiv-2021-9gxpp |
| [59] |
(a) Ashenhurst, J. A. Chem. Soc. Rev. 2010, 39, 540.
doi: 10.1039/b907809f pmid: 20111778 |
|
(b) Yang, Y.; Lan, J.; You, J. Chem. Rev. 2017, 117, 8787.
doi: 10.1021/acs.chemrev.6b00567 pmid: 20111778 |
|
| [60] |
(a) Simmons, R. L.; Yu, R. T.; Myers, A. G. J. Am. Chem. Soc. 2011, 133, 15870.
doi: 10.1021/ja206339s |
|
(b) Jbara, M.; Maity, S. K.; Brik, A. Angew. Chem., Int. Ed. 2017, 56, 10644.
doi: 10.1002/anie.201702370 |
|
|
(c) Bai, Z.; Cai, C.; Sheng, W.; Ren, Y.; Wang, H. Angew. Chem., Int. Ed. 2020, 59, 14686.
doi: 10.1002/anie.202007226 |
|
|
(d) Zhang, X.; Lu, G.; Sun, M.; Mahankali, M.; Ma, Y.; Zhang, M.; Hua, W.; Hu, Y.; Wang, Q.; Chen, J.; He, G.; Qi, X.; Shen, W.; Liu, P.; Chen, G. Nat. Chem. 2018, 10, 540.
doi: 10.1038/s41557-018-0006-y |
|
| [61] |
Kundu, R.; Ball, Z. T. Chem. Commun. 2013, 49, 4166.
doi: 10.1039/C2CC37323H |
| [62] |
Vinogradova, E. V.; Zhang, C.; Spokoyny, A. M.; Pentelute, B. L.; Buchwald, S. L. Nature 2015, 526, 687.
doi: 10.1038/nature15739 |
| [63] |
Rojas, A. J.; Zhang, C.; Vinogradova, E. V.; Buchwald, N. H.; Reilly, J.; Pentelute, B. L.; Buchwald, S. L. Chem. Sci. 2017, 8, 4257.
doi: 10.1039/C6SC05454D |
| [64] |
Rojas, A. J.; Pentelute, B. L.; Buchwald, S. L. Org. Lett. 2017, 19, 4263.
doi: 10.1021/acs.orglett.7b01911 |
| [65] |
Jbara, M.; Pomplun, S.; Schissel, C. K.; Hawken, S. W.; Boija, A.; Klein, I.; Rodriguez, J.; Buchwald, S. L.; Pentelute, B. L. J. Am. Chem. Soc. 2021, 143, 11788.
doi: 10.1021/jacs.1c05666 |
| [66] |
Messina, M. S.; Stauber, J. M.; Waddington, M. A.; Rheingold, A. L.; Maynard, H. D.; Spokoyny, A. M., J. Am. Chem. Soc. 2018, 140, 7065.
doi: 10.1021/jacs.8b04115 pmid: 29790740 |
| [67] |
Kulkarni, S. S.; Sayers, J.; Premdjee, B.; Payne, R. J. Nat. Rev. Chem. 2018, 2, 0122.
doi: 10.1038/s41570-018-0122 |
| [68] |
Li, F.; Allahverdi, A.; Yang, R.; Lua, G. B. J.; Zhang, X.; Cao, Y.; Korolev, N.; Nordenskiöld, L.; Liu, C.-F. Angew. Chem., Int. Ed. 2011, 50, 9611.
doi: 10.1002/anie.201103754 |
| [69] |
Arumugam, S.; Guo, J.; Mbua, N. E.; Friscourt, F.; Lin, N.; Nekongo, E.; Boons, G.-J.; Popik, V. V. Chem. Sci. 2014, 5, 1591.
doi: 10.1039/C3SC51691A |
| [70] |
(a) Hu, K.; Geng, H.; Zhang, Q.; Liu, Q.; Xie, M.; Sun, C.; Li, W.; Lin, H.; Jiang, F.; Wang, T.; Wu, Y.-D.; Li, Z. Angew. Chem., Int. Ed. 2016, 55, 8013.
doi: 10.1002/anie.201602806 |
|
(b) Tian, Y.; Li, J.; Zhao, H.; Zeng, X.; Wang, D.; Liu, Q.; Niu, X.; Huang, X.; Xu, N.; Li, Z. Chem. Sci. 2016, 7, 3325.
doi: 10.1039/C6SC00106H |
|
| [71] |
Zhao, G.; Kaur, S.; Wang, T. Org. Lett. 2017, 19, 3291.
doi: 10.1021/acs.orglett.7b01441 |
| [72] |
Vara, B. A.; Li, X.; Berritt, S.; Walters, C. R.; Petersson, E. J.; Molander, G. A. Chem. Sci. 2018, 9, 336.
doi: 10.1039/C7SC04292B |
| [73] |
Beard, H. A.; Hauser, J. R.; Walko, M.; George, R. M.; Wilson, A. J.; Bon, R. S. Commun. Chem. 2019, 2, 133.
doi: 10.1038/s42004-019-0235-z |
| [74] |
Bulaj, G. Biotechnol. Adv. 2005, 23, 87.
doi: 10.1016/j.biotechadv.2004.09.002 |
| [75] |
(a) Chalker, J. M.; Bernardes, G. J. L.; Davis, B. G. Acc. Chem. Res. 2011, 44, 730.
doi: 10.1021/ar200056q pmid: 22173886 |
|
(b) van Kasteren, S. Biochem. Soc. Trans. 2012, 40, 929.
doi: 10.1042/BST20120144 pmid: 22173886 |
|
|
(c) Bernardes, G. J. L.; Casi, G.; Trissel, S.; Hartmann, I.; Schwager, K.; Scheuermann, J.; Neri, D. Angew. Chem., Int. Ed. 2012, 51, 941.
doi: 10.1002/anie.201106527 pmid: 22173886 |
|
|
(d) List, T.; Casi, G.; Neri, D. Mol. Cancer Ther. 2014, 13, 2641.
doi: 10.1158/1535-7163.MCT-14-0599 pmid: 22173886 |
|
| [76] |
Dawson, P. E.; Muir, T. W.; Clarklewis, I.; Kent, S. B. H. Science 1994, 266, 776.
pmid: 7973629 |
| [77] |
Faustino, H.; Silva, M. J. S. A.; Veiros, L. F.; Bernardes, G. J. L.; Gois, P. M. P. Chem. Sci. 2016, 7, 6280.
doi: 10.1039/c6sc90045c pmid: 30124674 |
| [78] |
Zheng, X.; Li, Z.; Gao, W.; Meng, X.; Li, X.; Luk, L. Y. P.; Zhao, Y.; Tsai, Y.-H.; Wu, C. J. Am. Chem. Soc. 2020, 142, 5097.
doi: 10.1021/jacs.9b11875 |
| [79] |
Wu, Y.; Li, C.; Fan, S.; Zhao, Y.; Wu, C. Bioconjugate Chem. 2021, 32, 2065.
doi: 10.1021/acs.bioconjchem.1c00378 |
| [80] |
King, T. A.; Mandrup Kandemir, J.; Walsh, S. J.; Spring, D. R., Chem. Soc. Rev. 2021, 50, 39.
doi: 10.1039/D0CS00344A |
| [81] |
Zhao, Z.; Shimon, D.; Metanis, N. J. Am. Chem. Soc. 2021, 143, 12817.
doi: 10.1021/jacs.1c06101 |
| [82] |
Verhoog, S.; Kee, C. W.; Wang, Y.; Khotavivattana, T.; Wilson, T. C.; Kersemans, V.; Smart, S.; Tredwell, M.; Davis, B. G.; Gouverneur, V. J. Am. Chem. Soc. 2018, 140, 1572.
doi: 10.1021/jacs.7b10227 pmid: 29301394 |
| [83] |
Deng, J.-R.; Chung, S.-F.; Leung, A. S.-L.; Yip, W.-M.; Yang, B.; Choi, M.-C.; Cui, J.-F.; Kung, K. K.-Y.; Zhang, Z.; Lo, K.-W.; Leung, Y.-C.; Wong, M.-K. Commun. Chem. 2019, 2, 93.
doi: 10.1038/s42004-019-0193-5 |
| [84] |
Wan, L.-Q.; Zhang, X.; Zou, Y.; Shi, R.; Cao, J.-G.; Xu, S.-Y.; Deng, L.-F.; Zhou, L.; Gong, Y.; Shu, X.; Lee, G. Y.; Ren, H.; Dai, L.; Qi, S.; Houk, K. N.; Niu, D. J. Am. Chem. Soc. 2021, 143, 11919.
doi: 10.1021/jacs.1c05156 |
| [85] |
Toda, N.; Asano, S.; Barbas III, C. F. Angew. Chem., Int. Ed. 2013, 52, 12592.
doi: 10.1002/anie.201306241 |
| [86] |
Boutureira, O.; Bernardes, G. J. L.; Fernández-González, M.; Anthony, D. C.; Davis, B. G. Angew. Chem., Int. Ed. 2012, 51, 1432.
doi: 10.1002/anie.201106658 pmid: 22213253 |
| [1] | 张莹珍, 江丹丹, 李娟华, 王菁菁, 刘昆明, 刘晋彪. 高选择性硒代半胱氨酸荧光探针的构建策略及成像[J]. 有机化学, 2024, 44(1): 41-53. |
| [2] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [3] | 刘飞冉, 敬静, 张小玲. 细胞器靶向型半胱氨酸荧光探针研究进展[J]. 有机化学, 2023, 43(6): 2053-2067. |
| [4] | 陈莉, 黎俊波, 陈杜刚. 生物硫醇荧光探针的研究进展[J]. 有机化学, 2021, 41(2): 611-623. |
| [5] | 任涵, 李茹祥, 陈志坚, 李莉莉, 王浩. 自组装多肽的修饰方法及其应用[J]. 有机化学, 2021, 41(10): 3983-3994. |
| [6] | 周小琴, 崔梦园, 贾程利, 杨敏, 吉民, 王鹏. 一种新型比率型荧光探针用于细胞内半胱氨酸的检测[J]. 有机化学, 2020, 40(8): 2502-2507. |
| [7] | 申有名, 谷标, 刘歆, 唐裕才, 李海涛. 一种基于苯并噻唑衍生物的高选择性比率型高半胱氨酸荧光探针及生物成像[J]. 有机化学, 2020, 40(8): 2442-2449. |
| [8] | 田庆, 陈双虎, 陈景龙, 刘蕊, 汪雨诗, 杨晓朋, 叶勇. 一种基于罗丹明类似物的Cys近红外荧光探针[J]. 有机化学, 2019, 39(7): 2089-2093. |
| [9] | 王军, 虎良军, 申婧, 姜吉泉, 郁科勇, 孙荣国. L-半胱氨酸可视化传感器/体系的研究进展[J]. 有机化学, 2018, 38(4): 760-774. |
| [10] | 张薇, 姚子健, 邓维. 不同支化结构的聚酰胺胺的合成、活性及其在基因载体中的应用[J]. 有机化学, 2018, 38(10): 2713-2719. |
| [11] | 李春涛, 王蒙蒙, 朱倩, 曹迁永. 聚乙二醇单甲醚修饰蒽化合物纯水相荧光识别Hg2+及半胱氨酸[J]. 有机化学, 2017, 37(6): 1443-1449. |
| [12] | 杨太群, 戴山, 秦翠芳, 黄科翰, 陈瑜婷, 潘海峰, 张三军, 张坤, 徐建华. 利用聚甲基乙烯基醚共聚马来酸银簇实现高半胱氨酸探测[J]. 有机化学, 2016, 36(4): 867-871. |
| [13] | 王胜清, 申世立, 张延如, 戴溪, 赵宝祥. 小分子生物硫醇荧光探针研究进展[J]. 有机化学, 2014, 34(9): 1717-1729. |
| [14] | 杨睿, 杨丽娟, 董四花, 王淑娟, 林军. (4R)-5,5-二甲基-4-苄硫甲基-2-噁唑烷酮的合成[J]. 有机化学, 2010, 30(01): 103-106. |
| [15] | 周佳栋; 曹 飞*; 张小龙; 杨 颖; 应汉杰; 韦 萍. 一种基于共保护策略合成谷胱甘肽的新方法[J]. 有机化学, 2009, 29(08): 1272-1277. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||