有机化学 ›› 2024, Vol. 44 ›› Issue (1): 242-250.DOI: 10.6023/cjoc202307003 上一篇 下一篇
研究论文
徐利军a,b, 李宗军c, 韩福社a,b,*( ), 高翔a,b,*(
), 高翔a,b,*( )
)
收稿日期:2023-07-08
修回日期:2023-08-27
发布日期:2023-09-08
基金资助:
Lijun Xua,b, Zongjun Lic, Fushe Hana,b( ), Xiang Gaoa,b(
), Xiang Gaoa,b( )
)
Received:2023-07-08
Revised:2023-08-27
Published:2023-09-08
Contact:
*E-mail: Supported by:文章分享

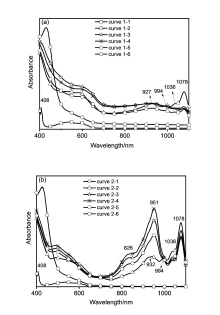
富勒烯稠合噁唑啉衍生物的合成已建立多种方法, 但绝大多数方法仅限于芳香族类底物. 发展了一种N,N-二甲基甲酰胺(DMF)促进富勒烯C60与酰胺类底物在碱性条件下合成富勒烯稠合噁唑啉的方法, 该方法对脂肪族和芳香族酰胺均具有较好的兼容性, 能以27%~62%的收率成功合成了一系列富勒烯稠合噁唑啉衍生物. 实验结果表明N,N-二甲基甲酰胺(DMF)在此反应中作为共溶剂, 可以显著提高目标化合物的收率. 现场可见近红外吸收光谱和对照实验研究表明, 富勒烯稠合噁唑啉二负离子是反应的关键中间体, DMF有利于此二负离子的生成并对此二负离子起到稳定作用. 电化学性质研究显示, 富勒烯稠合噁唑啉产物的噁唑啉环上不含取代基和含芳基取代基的衍生物, 其起始还原电位相对于C60无明显变化, 但噁唑啉环上含脂肪烷基的衍生物的起始还原电位有较大负移, 达到0.1 V.
徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250.
Lijun Xu, Zongjun Li, Fushe Han, Xiang Gao. N,N-Dimethylformamide-Promoted Synthesis of Fullerene-Fused Oxazoline Derivatives[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 242-250.
| Entry | Amide (1a)/equiv. | Solvent | TBAOH/equiv. | I2/equiv. | Temp./℃ | Time/h | Yieldb/% of 2a |
|---|---|---|---|---|---|---|---|
| 1 | 80 | o-DCB | 3 | 2 | 30 | 2 | 0 |
| 2 | 80 | V(o-DCB)∶V(DMF)=1∶1 | 3 | 2 | 30 | 2 | Trace |
| 3 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 3 | 2 | 30 | 2 | 9 |
| 4 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 2 | 30 | 2 | 13 |
| 5 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 2 | 30 | 2 | Trace |
| 6 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 5 | 30 | 2 | 13 |
| 7 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 5 | 30 | 2 | 17 |
| 8 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 30 | 2 | 19 |
| 9 | 80 | V(o-DCB)∶V(CH3CN)=3∶1 | 10 | 10 | 30 | 2 | 0 |
| 10 | 80 | V(o-DCB)∶V(THF)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 11 | 80 | V(o-DCB)∶V(DMSO)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 12 | 80 | V(o-DCB)∶V(Toluene)=3∶1 | 10 | 10 | 30 | 2 | 3 |
| 13 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 2 | 25 |
| 14 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 90 | 2 | 0 |
| 15 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| 16 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 0.5 | 23 |
| 17 | 40 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 25 |
| 18 | 100 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| Entry | Amide (1a)/equiv. | Solvent | TBAOH/equiv. | I2/equiv. | Temp./℃ | Time/h | Yieldb/% of 2a |
|---|---|---|---|---|---|---|---|
| 1 | 80 | o-DCB | 3 | 2 | 30 | 2 | 0 |
| 2 | 80 | V(o-DCB)∶V(DMF)=1∶1 | 3 | 2 | 30 | 2 | Trace |
| 3 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 3 | 2 | 30 | 2 | 9 |
| 4 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 2 | 30 | 2 | 13 |
| 5 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 2 | 30 | 2 | Trace |
| 6 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 5 | 30 | 2 | 13 |
| 7 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 5 | 30 | 2 | 17 |
| 8 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 30 | 2 | 19 |
| 9 | 80 | V(o-DCB)∶V(CH3CN)=3∶1 | 10 | 10 | 30 | 2 | 0 |
| 10 | 80 | V(o-DCB)∶V(THF)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 11 | 80 | V(o-DCB)∶V(DMSO)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 12 | 80 | V(o-DCB)∶V(Toluene)=3∶1 | 10 | 10 | 30 | 2 | 3 |
| 13 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 2 | 25 |
| 14 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 90 | 2 | 0 |
| 15 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| 16 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 0.5 | 23 |
| 17 | 40 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 25 |
| 18 | 100 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |



| Compound | E1red a/V | εLUMOb/eV |
|---|---|---|
| 2a | -0.99 | -3.81 |
| 2c | -1.07 | -3.73 |
| 2f | -1.01 | -3.79 |
| 2g | -1.00 | -3.80 |
| 2i | -1.01 | -3.79 |
| 2j | -0.99 | -3.81 |
| C60 | -0.97 | -3.83 |
| Compound | E1red a/V | εLUMOb/eV |
|---|---|---|
| 2a | -0.99 | -3.81 |
| 2c | -1.07 | -3.73 |
| 2f | -1.01 | -3.79 |
| 2g | -1.00 | -3.80 |
| 2i | -1.01 | -3.79 |
| 2j | -0.99 | -3.81 |
| C60 | -0.97 | -3.83 |
| [1] |
Zarrabi, N.; Seetharaman, S.; Chaudhuri, S.; Holzer, N.; Batista, V. S.; Van Der Est, A.; D’Souza, F.; Poddutoori, P. K. J. Am. Chem. Soc. 2020, 142, 10008.
doi: 10.1021/jacs.0c01574 |
| [2] |
Sun, C.; Yang, P.-P.; Nan, Z.; Tian, C.-B; Cai, Y.-T.; Chen, J.-F.; Qi, F.-F.; Tian, H.-R.; Xie, L.-Q.; Meng, L.-Y.; Wei, Z.-H. Adv. Mater. 2023, 35, 2205603.
doi: 10.1002/adma.v35.9 |
| [3] |
Li, S.-H.; Xing, Z.; Wu, B.-S.; Chen, Z.-C.; Yao, Y.-R.; Tian, H.-R.; Li, M.-F.; Yun, D.-Q.; Deng, L.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. ACS Appl. Mater. Interfaces 2020, 12, 20733.
doi: 10.1021/acsami.0c02119 |
| [4] |
Fan, J.-Q.; Fang, G.; Zeng, F.; Wang, X.-D.; Wu, S.-Z. Small 2013, 9, 613.
doi: 10.1002/smll.v9.4 |
| [5] |
Li, Y.-B.; Biswas, R.; Kopcha, W. P.; Dubroca, T.; Abella, L.; Sun, Y.; Crichton, R. A.; Rathnam, C.; Yang, L.-T.; Yeh, Y.-W.; Kundu, K.; Rodríguez-Fortea, A.; Poblet, J. M.; Lee, K.-B.; Hill, S.; Zhang, J.-Y. Angew. Chem., Int. Ed. 2023, 62, e202211704.
|
| [6] |
Ramos-Soriano, J.; Reina, J. J.; Illescas, B. M.; de la Cruz, N.; Rodríguez-Pérez, L.; Lasala, F.; Rojo, J.; Delgado, R.; Martín, N. J. Am. Chem. Soc. 2019, 141, 15403.
doi: 10.1021/jacs.9b08003 |
| [7] |
Zhu, S.-E.; Dou, L.-F.; Zhang, J.-H.; Wu, Y.; Yang, W.; Lu, H.-D.; Wei, C.-X.; Deng, C.-H.; Dong, Q. Chin. J. Org. Chem. 2021, 41, 2082 (in Chinese).
doi: 10.1002/cjoc.v41.17 |
|
(朱三娥, 豆礼锋, 张建辉, 吴缨, 杨伟, 鲁红典, 卫春祥, 邓崇海, 董强, 有机化学, 2021, 41, 2082.)
|
|
| [8] |
Niu, C.; Liu, Z.; Chen, M.-Q.; Yang, S.-F.; Wang, G.-W. Org. Lett. 2022, 24, 3493.
doi: 10.1021/acs.orglett.2c01097 |
| [9] |
Avila, L. B.; Serrano Arambulo, P. C.; Dantas, A.; Cuevas-Arizaca, E. E.; Kumar, D.; Müller, C. K. Nanomaterials 2022, 12, 2881.
doi: 10.3390/nano12162881 |
| [10] |
Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Sci. Bull. 2022, 67, 2406.
doi: 10.1016/j.scib.2022.11.023 |
| [11] |
Niu, C.; Wang, G.-W. Chin. J. Org. Chem. 2020, 40, 3633 (in Chinese).
doi: 10.6023/cjoc202006081 |
|
(牛闯, 王官武, 有机化学, 2020, 40, 3633.)
|
|
| [12] |
Hashiguchi, M.; Obata, N.; Maruyama, M.; Yeo, K. S.; Ueno, T.; Ikebe, T.; Takahashi, I.; Matsuo, Y. Org. Lett. 2012, 14, 3276.
doi: 10.1021/ol301186u |
| [13] |
Thiesbrummel, J.; Peña-Camargo, F.; Brinkmann, K. O.; Gutierrez- Partida, E.; Yang, F.-J.; Warby, J.; Albrecht, S.; Neher, D.; Riedl, T.; Snaith, H. J.; Stolterfoht, M.; Lang, F. Adv. Energy Mater. 2023, 13, 2202674.
doi: 10.1002/aenm.v13.3 |
| [14] |
Guo, Y.; Zhu, H.-X.; Liu, G.-L.; Yan, H.-M.; Zhu, B.-J.; Li, S.; Sun, Y.-J.; Li, G.-H. Chin. J. Org. Chem. 2016, 36, 172 (in Chinese).
|
|
(郭颖, 朱华新, 刘桂林, 严慧敏, 朱冰洁, 李帅, 孙亚军, 李果华, 有机化学, 2016, 36, 172.)
|
|
| [15] |
(a) Yang, W.-W.; Li, Z.-J.; Li, F.-F.; Gao, X. J. Org. Chem. 2011, 76, 1384.
doi: 10.1021/jo1023798 |
|
(b) Li, Z.-J.; Li, S.-H.; Sun, T.; Hou, H.-L.; Gao, X. J. Org. Chem. 2015, 80, 3566.
doi: 10.1021/acs.joc.5b00253 |
|
|
(c) Liu, Q.-S.; Qiu, W.-J.; Lu, W.-Q.; Wang, G.-W. Org. Biomol. Chem. 2022, 20, 3535.
doi: 10.1039/D2OB00239F |
|
| [16] |
(a) Banks, M. R.; Cadogan, J. I. G.; Gosney, I.; Hodgson, P. K. G.; Langridge-Smith, P. R. R.; Rankine, D. W. H. J. Chem. Soc., Chem. Commun. 1994, 25, 1365.
|
|
(b) Banks, M. R.; Cadogan, J. I. G.; Gosney, I.; Hodgson, P. K. G.; Langridge-Smith, P. R. R.; Millar, J. R. A.; Taylor, A. T. Tetrahedron Lett. 1994, 35, 9067.
doi: 10.1016/0040-4039(94)88429-3 |
|
| [17] |
Averdung, J.; Mattay, J.; Jacobi, D.; Abraham, W. Tetrahedron 1995, 51, 2543.
doi: 10.1016/0040-4020(95)00013-X |
| [18] |
Yang, H.-T.; Xing, M.-L.; Zhu, Y.-F.; Sun, X.-Q.; Cheng, J.; Miao, C.-B.; Li, F.-B. J. Org. Chem. 2014, 79, 1487.
doi: 10.1021/jo4025573 |
| [19] |
(a) Zheng, M.; Li, F.-F.; Ni, L.; Yang, W.-W.; Gao, X. J. Org. Chem. 2008, 73, 3159.
doi: 10.1021/jo702678c |
|
(b) Hou, H.-L. Gao, X. J. Org. Chem. 2012, 77, 2553.
doi: 10.1021/jo202616j |
|
| [20] |
Chang, W.-W.; Li, Z.-J.; Yang, W.-W.; Gao, X. Org. Lett. 2012, 14, 2386.
doi: 10.1021/ol300805p |
| [21] |
(a) Li, F.-B.; Liu, T.-X.; Wang, G.-W. J. Org. Chem. 2008, 73, 6417.
doi: 10.1021/jo8007868 |
|
(b) Yang, H.-T.; Liang, X.-C.; Wang, Y.-H.; Yang, Y.; Sun, X.-Q.; Miao, C.-B. Org. Lett. 2013, 15, 4650.
doi: 10.1021/ol401909z |
|
|
(c) Zhang, X.-F.; Li, F.-B.; Shi, J.-L.; Wu, J.; Liu, L. New J. Chem. 2016, 40, 1626.
doi: 10.1039/C5NJ02503F |
|
|
(d) Liu, T.-X.; Liu, Y.-Q.; Chao, D.; Zhang, P.-L.; Liu, Q.-F.; Shi, L.; Zhang, Z.-G.; Zhang, G.-S. J. Org. Chem. 2014, 79, 11084.
doi: 10.1021/jo5020883 |
|
|
(e) Liu, Q.-S.; Qiu, W.-J.; Lu, W.-Q.; Wang, G.-W. Org. Biomol. Chem. 2022, 20, 3535.
doi: 10.1039/D2OB00239F |
|
| [22] |
Takeda, Y.; Enokijima, S.; Nagamachi, T.; Nakayama, K.; Minakata, S. Asian J. Org. Chem. 2013, 2, 91.
doi: 10.1002/ajoc.v2.1 |
| [23] |
Yang, H.-T.; Ren, W.-L.; Dong, C.-P.; Yang, Y.; Sun, X.-Q.; Miao, C.-B. Tetrahedron Lett. 2013, 54, 6799.
doi: 10.1016/j.tetlet.2013.09.002 |
| [24] |
Rosén, A.; Wästberg, B. J. Chem. Phys. 1989, 90, 2525.
doi: 10.1063/1.455947 |
| [25] |
Xie, Q.-S.; Perez-Cordero, E.; Echegoyen, L. J. Am. Chem. Soc. 1992, 114, 3978.
doi: 10.1021/ja00036a056 |
| [26] |
(a) Fagan, P. J.; Krusic, P. J.; Evans, D. H.; Lerke, S. A.; Johnston, E. J. Am. Chem. Soc. 1992, 114, 9697.
doi: 10.1021/ja00050a081 |
|
(b) Schick, G.; Kampe, K. D.; Hirsch, A. J. Chem. Soc., Chem. Commun. 1995, 19, 2023.
|
|
|
(c) Wang, G.-W.; Shu, L.-H.; Wu, S.-H.; Wu, H.-M.; Lao, X.-F. J. Chem. Soc., Chem. Commun. 1995, 10, 1071.
|
|
| [27] |
Matsuo, Y.; Iwashita, A.; Abe, Y.; Li, C.-Z.; Matsuo, K.; Hashiguchi, M.; Nakamura, E. J. Am. Chem. Soc. 2008, 130, 15429.
doi: 10.1021/ja8041299 |
| [28] |
Isobe, H.; Tanaka, T.; Nakanishi, W.; Lemiègre, L.; Nakamura, E. J. Org. Chem. 2005, 70, 4826.
doi: 10.1021/jo050432y |
| [29] |
Chang, W.-W.; He, F.-G.; Garcıa-Penas, A.; Shekh, M. I.; Li, Z.-J. RSC Adv. 2022, 12, 14018.
doi: 10.1039/D2RA01300B |
| [30] |
Naim, A.; Shevlin, P. B. Tetrahedron Lett. 1992, 33, 7097.
doi: 10.1016/S0040-4039(00)60845-6 |
| [31] |
Hare, J. P.; Kroto, H. W.; Taylor, R. Chem. Phys. Lett. 1991, 177, 394.
doi: 10.1016/0009-2614(91)85072-5 |
| [32] |
Khaled, M. M.; Carlin, R. T.; Trulove, P. C.; Eaton, G. R.; Eaton, S. S. J. Am. Chem. Soc. 1994, 116, 3465.
doi: 10.1021/ja00087a037 |
| [33] |
Rapta, P.; Bartl, A.; Gromov, A.; Stasko, A.; Dunsch, L. ChemPhyChem 2002, 3, 351.
|
| [34] |
Subramanian, R.; Kadish, K. M.; Vijayashree, M. N.; Gao, X.; Jones, M. T.; Miller, M. D.; Krause, K. L.; Suenobu, T.; Fukuzumi, S. J. Phys. Chem. 1996, 100, 16327.
doi: 10.1021/jp961412q |
| [35] |
Xu, L.-J.; Yang, W.-W.; Han, F.-S.; Gao, X. Org. Biomol. Chem. 2023, 21, 2331.
doi: 10.1039/D3OB00039G |
| [36] |
(a) Li, F.-F.; Gao, X.; Zheng, M. J. Org. Chem. 2009, 74, 82.
doi: 10.1021/jo801769q |
|
(b) Yang, W.-W.; Li, Z.-J.; Li, S.-H.; Gao, X. J. Phys. Chem. A 2015, 119, 9534.
doi: 10.1021/acs.jpca.5b07932 |
|
| [37] |
(a) Liu, Z.-J.; Larock, R. C. J. Am. Chem. Soc. 2005, 127, 13112.
doi: 10.1021/ja054079p |
|
(b) Sukata, K. Bull. Chem. Soc. Jpn. 1985, 58, 838.
doi: 10.1246/bcsj.58.838 |
|
| [38] |
Zhao, H.; Zhu, X.-Y.; Hu, X.-X.; Liu, Y.-G.; Tang, C.-L.; Feng, B.-N. Chin. J. Org. Chem. 2019, 39, 434 (in Chinese).
doi: 10.6023/cjoc201807010 |
|
(赵辉, 朱孝云, 胡小霞, 刘延革, 唐春雷, 冯柏年, 有机化学, 2019, 39, 434.)
|
|
| [39] |
Hou, H.-L.; Li, Z.-J.; Li, S.-H.; Chen, S.; Gao, X. Org. Lett. 2013, 15, 4646.
doi: 10.1021/ol401834j |
| [40] |
Zhuo, L.-G.; Liao, W.; Yu, Z.-X. Asian J. Org. Chem. 2012, 1, 336.
doi: 10.1002/ajoc.v1.4 |
| [41] |
(a) Li, D.; Li, Z.-J.; He, F.-G.; Geng, C.; Gao, X. J. Org. Chem. 2019, 84, 14679.
doi: 10.1021/acs.joc.9b02272 |
|
(b) Yang, S.-T.; Zhou, X.-Y.; Hu, Y.-J.; Abella, L.; Yao, Y.-R.; Peng, P.; Zhang, Q.-Y.; Rodríguez-Fortea, A.; Poblet, J. M.; Li, F.-F. J. Org. Chem. 2023, 88, 4234.
doi: 10.1021/acs.joc.2c02779 |
|
| [42] |
Li, X.-J.; Li, Y.-F. Acta Polym. Sin. 2022, 53, 995 (in Chinese).
|
|
(李骁骏, 李永舫, 高分子学报, 2022, 53, 995.)
|
| [1] | 胡朝明, 吴纪红, 吴晶晶, 吴范宏. 直接三氟甲硒基化反应研究进展[J]. 有机化学, 2023, 43(1): 36-56. |
| [2] | 张广宇, 许家喜. 羟胺衍生物的[3,3] σ迁移反应及其应用[J]. 有机化学, 2021, 41(8): 3002-3014. |
| [3] | 袁文豪, 许家喜. 氧杂环丁烷的扩环反应[J]. 有机化学, 2021, 41(3): 947-958. |
| [4] | 陶雪芬, 盛荣, 鲍堃, 王玉新, 金银秀. 二氟甲基杂芳基砜作为二氟烷基化试剂的应用研究进展[J]. 有机化学, 2019, 39(10): 2726-2734. |
| [5] | 李标, 刘秋霞, 周元清, 贾赵栋, 朱曼毓, 徐琰, 宋毛平. 二茂铁苯甲酸香豆素类化合物的合成与性质研究[J]. 有机化学, 2017, 37(8): 2008-2014. |
| [6] | 任亚平, 刘絮, 王瑞, 周元清, 李标, 徐琰, 宋毛平. 对二茂铁苯甲酰噻二唑类化合物的合成和性质研究[J]. 有机化学, 2017, 37(1): 110-115. |
| [7] | 黄敏, 娄兆文, 彭小倩, 何汉平, 张修华, 王升富. 一类二茂铁衍生物的合成及其对铬离子和铜离子的特异性识别研究[J]. 有机化学, 2015, 35(9): 1966-1974. |
| [8] | 吴孔丽, 张吾斌, 周丹, 徐琰. 二茂铁甲酰基丙酸二肽的合成与电化学性质的研究[J]. 有机化学, 2014, 34(6): 1201-1205. |
| [9] | 闾新明, 钱鹰. 新型超支化荧光聚合物的合成及光物理性质[J]. 有机化学, 2011, 31(01): 82-86. |
| [10] | 肖 玲 ; 曾和平*. N-甲基-2-{N-乙基-6-[2-(8-甲氧基喹啉基)乙烯基]咔唑基}富勒烯 吡咯烷的合成、电化学性质与双光子吸收特性[J]. 有机化学, 2009, 29(05): 742-747. |
| [11] | 崔大军,彭立军,曾宪顺,徐风波,张正之. 制备trans-Fe(CO)_3(PR_3)_2的新的取代过程[J]. 有机化学, 2003, 23(9): 973-976. |
| [12] | 阮继武,黄忠京,符立梧,马林,古练权. 胺类化合物与4,4’-二氟苯偶酰的亲核取代反应研究[J]. 有机化学, 2003, 23(8): 861-864. |
| [13] | 宣光荣,何宁德. 7,7-二氧双环[4,1,0]庚烷与活性亚甲基化合物的反应[J]. 有机化学, 2003, 23(7): 734-736. |
| [14] | 王建武,贾炯,候殿杰,李红梅,尹军. 一个新的制备咪唑并[1,5-α]吡啶衍生物的串联反应[J]. 有机化学, 2003, 23(2): 173-175. |
| [15] | 张素娜,于建新,李中军,孙万赋,周蓉,张丽静,王瑞英,蔡孟深. 6-S-(取代的三或四唑杂环基)-1,2:3,4-二-O-异亚丙基-α-D吡喃型半乳糖的合成研究[J]. 有机化学, 2003, 23(2): 176-181. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||