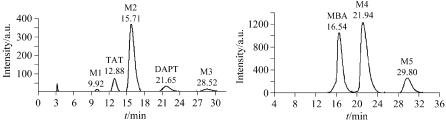
1,3,5,7-四乙酰基-1,3,5,7-四氮杂环辛烷(TAT)是高能炸药奥克托今(HMX)重要的合成反应前体之一, 1,5-二乙酰基-1,3,5,7-四氮杂环辛烷(DAPT)醋酐酰解法和乙腈甲醛小分子缩合法均能制备得到TAT. 采用半制备高效液相色谱制备分离了上述两种制备方法反应原液中的六种中间体, 分别是1-乙酰氧甲基-3,5,7-三乙酰基-1,3,5,7-四氮杂环辛烷、1,3,5-三乙酰基-1,3,5,7-四氮杂环辛烷、1-乙酰氧甲基-3,5,7-三乙酰基-1,3,5,7-四氮杂环辛烷、N,N'-亚甲基二乙酰胺、三乙酰胺二亚甲基三胺和四乙酰胺三亚甲基四胺, 为反应机理研究提供了有力的证据. DAPT醋酐酰解法反应原液样品的最佳制备色谱条件为: 正相硅胶柱(20 mm×250 mm, 10~20 μm), 流动相: V(乙腈)∶V(甲醇)=95∶5, 流速为10mL·min-1, 检测波长为215 nm, 进样量为1 mL, 样品浓度30 mg/mL. 乙腈甲醛小分子缩合法反应原液样品的最佳制备色谱条件为: 正相硅胶柱(20 mm×250 mm, 10~20 μm), 流动相: V(乙腈)∶V(水)=90∶10, 流速为10 mL·min-1, 检测波长为215 nm, 进样量为1 mL, 样品浓度30 mg/mL. 制备得到的中间体经HPLC 分析知纯度均在97%以上, 可直接作为结构鉴定的标准样品.
李清霞
,
王鹏
,
娄忠良
,
刘越
,
孟子晖
,
徐志斌
,
薛敏
. 1,3,5,7-四乙酰基-1,3,5,7-四氮杂环辛烷合成反应中间体的制备分离[J]. 有机化学, 2012
, 32(01)
: 165
-168
.
DOI: 10.6023/cjoc1106091
1,3,5,7-Tetraacetyl-1,3,5,7-tetraazacyclooctane (TAT) was a key intermediates for the synthesis of 1,3,5,7-tetranitro- 1,3,5,7-tetrazocine (HMX) that is a highly energetic material. TAT can be prepared from the acetylation of 3,7-diacetyl- 1,3,5,7-tetraazacyclooctane (DAPT) and the small molecules condensation reaction. In this paper, sem preparative high performance piquid chromatography (SPHPLC) was used as a new separation and purification method for the intermediates in the reaction solution, and six samples obtained with high purity (>97%) analyzed by HPLC could prove much evidence and information for the reaction mechanism research. The optimum technological parameters of SPHPLC as follows: Si column (20 mm×250 mm, 10~20 μm), V(acetonitrile)∶V(water)=95∶5 (for the method of the acetylation of DAPT), V(acetonitrile)∶ V(methanol)=90∶10 (for the method of the small molecules condensation reaction), 10 mL·min-1, 215 nm, 1 mL (30 mg· mL-1).
[1] Agrawal, J. P.; Hodgson, R. D. Textbook of Organic Chemistry of Explosives, John Wiley and Sons. Ltd, England, 2007, pp. 247~ 261.
[2] Cooney, A. P.; Crampton, M. R.; Jones, M.; Golding, P. J. Heterocycl. Chem. 1987, 24, 1163.
[3] Agadzhanyan, T. E.; Minasyan, G. G. Arm. Khim. Zh. 1982, 35, 315.
[4] Siele, V. I.; Warman, M.; Gilbert, E. E. J. Heterocycl. Chem. 1974, 11, 237.
[5] Lukasavage, W. J.; Behrmann, L. A.; Voreck, W. E. WO 2000073 285, 2000 [Chem. Abstr. 1983, 134, 861664].
[6] Wang, P.; Meng, Z.-H.; Li, J.-M.; Lou, Z.-L.; Xu, Z.-B. CN 101863849, 2010 [Chem. Abstr. 2010, 153, 622415].
[7] Zhang, Y.; Jiao, J.-J.; Liu, C.-M. Food Chem. 2008, 107, 1326.
[8] Nazemiyeh, H.; Rahman, M. M.; Gibbons, S. J. Nat. Med. 2008, 62, 91.
[9] Hagelin, C.; Oulie, I.; Raknes, A. J. Chromatogr. B 2004, 811, 243.
[10] Lou, Z.-L.; Meng, Z.-H.; Meng, W.-J.; Wang. P. Chin. J. Energ. Mater. 2010, 18, 226 (in Chinese). (娄忠良, 孟子晖, 孟文君, 王鹏, 含能材料, 2010, 18, 226.)
[11] Song, H.-Y.; Wang, P.; Qin, G.-M. Chin. J. Org. Chem. 2010, 30, 414 (in Chinese). (宋红燕, 王鹏, 覃光明, 有机化学, 2010, 30, 414.)
[12] Li, Q.-X.; Wang, P.; Meng, W.-J. Chin. J. Org. Chem. 2009, 29, 409 (in Chinese). (李清霞, 王鹏, 孟文君, 孟子晖, 有机化学, 2009, 30, 409.)
[13] Lou, Z.-L.; Wang, P.; Meng, Z.-H. Chin. J. Org. Chem. 2010, 30, 1059 (in Chinese). (娄忠良, 王鹏, 孟子晖, 有机化学, 2010, 30, 1059.)