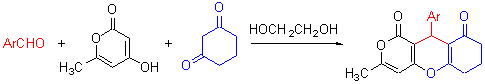
以芳醛、4-羟基-6-甲基-2-吡喃酮和1,3-环己二酮为原料, 乙二醇为溶剂合成了一系列3-甲基-9-芳基-1,8-二氧代-2,10-二氧杂蒽衍生物. 该反应产率高(70%~83%)、操作简单、后处理方便. 产物的结构经红外光谱、核磁共振谱及单晶X 射线衍射法表征.
游新奎
,
林志兰
,
张雪利
,
高原
. 3-甲基-9-芳基-1,8-二氧代-2,10-二氧杂蒽衍生物的合成[J]. 有机化学, 2012
, 32(01)
: 156
-159
.
DOI: 10.6023/cjoc1104071
A series of 9-aryl-3-methyl-1,8-dioxo-2,10-dioxanthens were synthesized by the reaction of aromatic aldehydes, 4-hydroxyl-6-methyl-2-pyranone and 1,3-cyclohexadione in glycol. The structures of the products were determined by IR, NMR and X-ray diffraction analysis.
[1] Wickenden, A. D. Pharmacol. Ther. 2002, 94, 157
[2] Brooks, G. T.; Ottridge, A. P.; Mace, D. W. Pestic. Sci. 1998, 22, 41.
[3] Witte, E. C.; Neubert, P.; Roesch, A. DE 3427985, 1986 [Chem. Abstr. 1986, 104, 224915f].
[4] Greenblatt, M. S.; Benntt, W. P.; Hollstein, M.; Harris, C. C. Cancer Res. 1994, 54(18), 4855.
[5] Hyama, T.; Saimoto, H. JP 62181276, 1987 [Chem. Abstr. 1988, 108, 37645].
[6] Chand, N.; Diamantis, W.; Sofia, R. D. Br. J. Pharmacol. 1986, 87(2), 443.
[7] (a) Suárez, M.; Verdecia, Y.; Ochoa, E.; Salfán, E.; Morán, L.; Martín, N.; Martíne, R.; Quinteiro, M.; Seoane, C.; Soto, J. L.; Novoa, H.; Blaton, N.; Peeters, O. M.; Ranter, C. D. Eur. J. Org. Chem. 2000, 2079. (b) Suárez, M.; Ochoa, E.; Verdecia, Y.; Martín, M.; Quinteiro, C.; Seoane, J.; Soto, J. L.; Novoa, H.; Blaton, N.; Peeters, O. M. Tetrahedron 1999, 55, 875.
[8] Ji, X. S.; Liu, Y.; Miao, Y.; Jin, T. Chin. J. Pharm. Sci. 1998, 7(4), 221 (in Chinese). (季小慎, 刘延, 苗弈, 金涛, 药理科学, 1998, 7(4), 221.)
[9] Zhao, T. F.; Zhao, D. F.; Sun, X. S.; Cheng, L. B. Chem. Ind. Eng. 1998, 49(4), 515 (in Chinese). (赵同丰, 赵德风, 孙孝森, 程侣柏, 化工学报, 1998, 49(4), 515.)
[10] Liang, B.; Kalidindi, S.; Porco, J. A. Jr.; Stephenson, C. R. J. Org. Lett. 2010, 12(3), 572.
[11] Li, M. H.; Chen, C.; He, F.; Gu, Y. L. Adv. Synth. Catal. 2010, 352, 519.