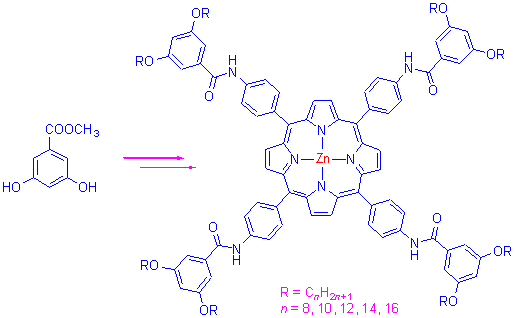
设计合成了5 种未见文献报道的5,10,15,20-四{对[3,5-二-(烷氧基)苯甲酰胺基]苯基}卟啉及其锌金属配合物, 并用IR, UV-Vis, 1H NMR, 元素分析以及XPS 对其组成和结构进行了表征, 研究了这10 种酰胺基系列卟啉化合物的拉曼光谱和荧光光谱的变化. 结果显示链长对荧光和拉曼光谱没有明显影响, 其取代基效应基本相同, 配体的荧光强度强于锌配合物的荧光强度. 在拉曼光谱中, 由于卟啉分子平面的对称性由D2h 变为D4h 群及其锌离子d 轨道的电子效应,卟啉配体和锌配合物之间的拉曼光谱有很大差别.
In this paper, five novel 5,10,15,20-tetra[4-(3,5-dialkoxybenzamide)phenyl]porphyrin ligands and their Zn complexes were synthesized and characterized by means of IR, UV-Vis, 1H NMR, elemental analysis and XPS. Raman and fluorescence spectra of the ten amide group porphyrin ligands and Zn complexes were investigated. The results show that the length of side chains does not have evident effect on the Raman spectra and fluorescence spectra of porphyrin compounds, their substituent effect is basically the same. The fluorescence intensity of porphyrin ligands was stronger than that of Zn complexes. In the Raman spectra, there were much difference between the porphyrin ligands and Zn complexes due to changes of the symmetry of porphyrin plane and electron effect of the d orbits of the zinc ion.
[1] Smith, K. M. Porphyrins and Metalloporphyrins, Amsterdan, Elsevier, 1975.
[2] Lammi, R. K.; Ambroise, A.; Balasubramanian, T.; Wagner, R. W.; Bocian, D. F.; Holten, D.; Lindsey, J. S.; J. Am. Chem. Soc. 2000, 122, 7579.
[3] Sternberg, E. D.; Dolphin, D.; Bruckner, C. Tetrahedron 1998, 54, 4151.
[4] Li, J.; Ambroise, A.; Yang, S. I. J. Am. Chem. Soc. 1999, 121, 8927.
[5] Anderson, H. L. Chem. Commun. 1999, 23, 2323.
[6] Burroughes, H.; Bradley, D. C.; Brown, A. R.; Parker, L. D. Nature 1990, 347, 539.
[7] (a) Ohtake, T.; Ogasawara, M.; Ito-Akita, K.; Nishina, N.; Ujiie, S.; Ohno, H. Chem. Mater. 2000, 12, 782. (b) Percec, V.; Johansson, G.; Ungar, G.; Zhou, J. J. Am. Chem. Soc. 1996, 118, 9855. (c) Lehmann, M.; Gearba, R. I.; Koch, M. H. J.; Ivanov, D. A.Chem. Mater. 2004, 16, 374.
[8] Zhu, B. K.; Xu, Z. K. ; Xu, Y. Y. Chin. J. Appl. Chem. 1999, 16(1), 68 (in Chinese). (朱宝库, 徐志康, 徐又一, 应用化学, 1999, 16(1), 68.)
[9] Wang, D. J.; Zhang, J.; Shi, T. S.; Wang, B. H. J. Photochem. Photobiol. A 1996, 93, 21.
[10] Horrocks Jr, W. D.; Wong, C.-P. J. Am. Chem. Soc. 1976, 98, 7157.
[11] Liu, W.; Shi, Y. H.; Shi, T. S. Chem. J. Chin. Univ. 2003, 24, 200.
[12] Maaon, S. F. J. Am. Chem. Soc. 1958, 976.
[13] Gouterman, M. J. Mol. Spectrosc. 1961, 6, 138
[14] Luo, G. T.; Xu, Q. B.; Chen, Z. X.; Teng, L. L. Chin. J. Org. Chem. 2009, 29, 9 (in Chinese). (罗国添, 徐庆兵, 陈治希, 滕莉丽, 有机化学, 2009, 29, 9.)
[15] Jiang, Y. S.; Li, T. J. Photochemistry, Chemical Industry Press, Beijing, 2005 (in Chinese). (姜月顺, 李铁津, 光化学, 化学工业出版社, 2005.)
[16] Chen, G. Z.; Huang, X. Z.; Zheng, Z. Z.; Xu, J. G.; Wang, Z. B.; Fluorometric Determination, Science Press, Beijing, 1990 (in Chinese). (陈国珍, 黄贤智, 郑朱梓, 许金钩, 王尊本, 荧光分析法, 科学 出版社, 北京, 1990.)
[17] Falk, E. Porphyrins and Metalloporphyrin, Elsevier, Amsterdan, 1964, p. 55.
[18] Gottfried, J. M.; Flechtner, K.; Kretschmann, A.; Lukasczyk, T.; Steinruck, H. P. J. Am. Chem. Soc. 2006, 128, 5644.
[19] Wagner, C. D.; Riggs, W. M.; Davis, L. E.; Moulder, J. F.; Muilenberg, G. E. Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, New York, 1979.