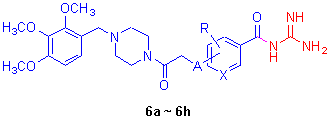
NHE1 (Na+/H+交换器1)抑制剂对于心肌缺血再灌注损伤具有较好的保护作用. 以苯(或吡啶)甲酰胍为母核, 利用拼合原理, 在苯(或吡啶)甲酰胍母环上引入4-(2,3,4-三甲氧基苄基)哌嗪-1-甲基, 设计并合成了8 个未见文献报道的目标化合物. 其结构经MS, IR, 1H NMR 和元素分析确证. 体外血小板肿胀模型(PSA)试验结果表明, 大部分目标化合物显示出较好的NHE1 抑制活性.
靳璐
,
姜向敏
,
何广卫
,
徐文婷
,
龚国清
,
徐云根
. 4(6)-{2-[4-(2,3,4-三甲氧基苄基)哌嗪-1-基]-2-氧代乙氧基}芳甲酰胍类化合物的合成及其Na+/H+交换器1 抑制活性[J]. 有机化学, 2012
, 32(02)
: 326
-332
.
DOI: 10.6023/cjoc1108051
Na+/H+ exchanger 1 (NHE1) inhibitors have a protective effect against myocardial ischemic-reperfusion injury. By choseing benzoyl (or pyridine-3-carbonyl) guanidine as main structure, and introducing 4-(2,3,4-trimethoxybenzyl)piperazine- 1-yl to its benzene (or pyridine) ring, eight compounds were designed and synthesized. All the target compounds have not been reported and their structures have been confirmed by IR, 1H NMR, MS and elemental analysis. The rat platelet swelling assay (PSA) results showed that most of the tested compounds exhibited potent NHE1 inhibitory effects.
[1] Malo, M. E.; Fliegel, L. Can. J. Physiol. Pharmacol. 2006, 84, 1081.
[2] Masereel, B.; Pochet, L.; Laeckmann, D. Eur. J. Med. Chem. 2003, 38, 547.
[3] Karmazyn, M.; Sawyer, M.; Fliegel, L. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 323.
[4] Park, H. S.; Lee, B. K.; Park, S.; Kim, S. U.; Lee, S. H.; Baik, E. J.; Lee, S.; Yi, K. Y.; Yoo, S. E.; Moon, C. H.; Jung, Y. S. Brain Res. 2005, 1061, 67.
[5] Zhang, R.; Lei, L.; Xu, Y. G.; Hua, W. Y.; Gong, G. Q. Bioorg. Med. Chem. Lett. 2007, 17, 2430.
[6] Jiang, X. M.; Du, P.; Xu, Y. G.; Zhang, D.; Gong, G. Q.; You, Q. D. Chin. J. Org. Chem. 2008, 28(12), 2142 (in Chinese). (姜向敏, 杜萍, 徐云根, 张迪, 尤启冬, 有机化学, 2008, 28(12), 2142.)
[7] Xu, W. T.; Jin, N.; Xu, J.; Xu, Y. G.; Wang, Q. J.; You, Q. D. Bioorg. Med. Chem. Lett. 2009, 19, 3283.
[8] Jin, L.; Jiang, X. M.; Du, P.; Xu, J.; Gong, G. Q.; Wang, Q. J.; Xu, Y. G. Eur. J. Med. Chem. 2011, 46, 4107.
[9] Rosskopf, D.; Morgenstern, E.; Scholz, W.; Osswald, U.; Siffert, W. J. Hypertens 1991, 9, 231.