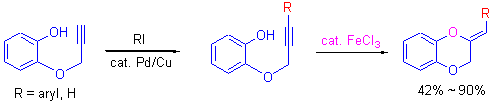
通过Sonogashira 偶联反应合成了邻炔丙基醚苯酚衍生物, 在Cs2CO3 存在和FeCl3 的催化下, 进而发生分子内环化反应, 立体选择性地合成了一系列(Z)-2-亚基-1,4-苯并二噁烷衍生物. 产物的结构通过了1H NMR, 13C NMR和HRMS等表征.
梁淋峰
,
方奇
,
徐晓冰
,
李艳忠
. 碳酸铯存在下铁催化立体选择性合成(Z)-2-亚基-1,4-苯并二噁烷衍生物[J]. 有机化学, 2012
, 32(02)
: 409
-412
.
DOI: 10.6023/cjoc1107111
A series of monopropargyl catechol derivatives synthesized via the Sonogashira coupling reaction were stereoselectively converted to (Z)-2-ylidene-1,4-benzodioxanes using FeCl3 as the catalyst, and structurally characterized by 1H NMR, 13C NMR and HRMS techniques.
[1] Merlini, L.; Zanarotti, A.; Pelter, A.; Haensel, R. J. Chem. Soc., Chem. Commun. 1979, 695.
[2] Arnone, A.; Merlini, L.; Zanarotti, A. J. Chem. Soc., Chem. Commun. 1979, 696.
[3] Bosseray, P.; Guillaumet, G.; Coudert, G.; Wasserman, H. Tetrahedron Lett. 1989, 30, 1387.
[4] Wang, Q. A.; Chen, Q. Q. Chin. J. Org. Chem. 2000, 20, 910 (in English). (汪秋安, 陈清奇, 有机化学, 2000, 20, 910.)
[5] Marciniak, G.; Delgado, A.; Leclerc, G.; Decker, N.; Schwartz, J. J. Med. Chem. 1989, 32, 1402.
[6] Ruffolo, Jr. R. R.; Bondinell, W.; Hieble, J. P. J. Med. Chem. 1995, 38, 3681.
[7] Quaglia, W.; Pigini, M.; Leornadi, A.; Taddei, C.; Melchiorre, C. J. Med. Chem. 1996, 39, 2253.
[8] Labrosse, J. R.; Lhoste, P.; Sinou, D. Tetrahedron Lett. 1999, 40, 9025.
[9] Labrosse, J. R.; Lhoste, P.; Sinou, D. Eur. J. Org. Chem. 2003, 2813.
[10] Dominczak, N.; Lhoste, P.; Kryczka, B.; Sinou, D. J. Mol. Catal. A: Chem. 2007, 264, 110.
[11] Chowdhury, C.; Chaudhury, G.; Guha, S.; Kundu, N. G. J. Org. Chem. 1998, 63, 1863.
[12] Basak, A.; Bhattacharya, G.; Mallik, U. K.; Khamrai, U. K. Synth. Commun. 1997, 27, 367.
[13] Yamamoto, M. J. Chem. Soc., Perkin Trans. 1 1979, 3161.
[14] Kalgutkar, A. S.; Kozak, K. R.; Crews, B. C.; Marnett, L. J. J. Med. Chem. 1998, 41, 4800.
[15] Yamamoto, M. J. Chem. Soc., Chem. Commun. 1978, 649.
[16] Arcadi, A.; Burini, A.; Cacchi, S.; Marinelli, F.; Pietroni, B. R. J. Org. Chem. 1992, 57, 976.