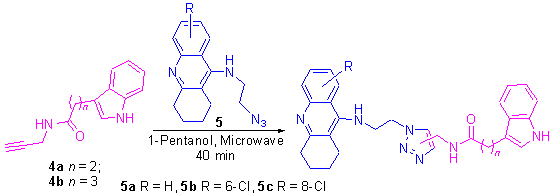
以吲哚-3-丙酸和吲哚-3-丁酸为原料, 分别与炔丙胺发生缩合反应得到3-(丙酰丙炔胺)吲哚(4a)和3-(丁酰丙炔胺)吲哚(4b), 然后4a 和4b 分别与9-(叠氮基乙基氨基)-1,2,3,4-四氢吖啶类衍生物5a~5c 在微波辐射下发生Husigen[3+2]环加成反应得到12 个新型乙酰胆碱酯酶抑制剂——他克林-吲哚杂二联体, 其结构经NMR, IR 和HRMS 表征.初步生物活性测试表明, 目标化合物均具有较强的乙酰胆碱酯酶抑制能力, 其中化合物2b 和2d 抑制鱼鳐乙酰胆碱酯酶的IC50 值分别为1.6 和2.0 nmol·L-1, 是6T6BA (IC50=11.0 nmol·L-1, 鱼鳐)的6.9 和5.5 倍.
郭永彪
,
刘海波
,
许明
. 新型他克林-吲哚杂二联体的微波促进Husigen [3+2]环加成反应合成及生物活性[J]. 有机化学, 2012
, 32(02)
: 413
-419
.
DOI: 10.6023/cjoc1105143
3-(Propargyl-propionamine)indole (4a) and 3-(propargyl-butyramide)indole (4b) were prepared from indole-3-propionic acid (3a) and indole-3-butyric acid (3b) by condensation with propargylamine, respectively. Twelve novel acetylcholinesterase inhibitors, tacrine-indole hybrids, were synthesized from 4a, 4b and 9-(azido-ethyl-amino)- 1,2,3,4-tetrahydroacridine derivatives (5a~5c) by microwave-assisted Huisgen [3+2] cycloaddition reaction. These novel compounds were determined by NMR, IR and HRMS techniques. The preliminary bioassay showed that all of them exhibited better AChE (electrophorus) inhibitory activity. Particularly, the IC50 values of compounds 2b and 2d were 1.6 and 2.0 nmol· L-1 respectively, which were 6.9 and 5.5 times respectively more potent than N-[6-(6-chloro-1,2,3,4-tetrahydroacridin-9- yl-amino)hexyl]-3-(1H-Indol-3-yl)propionamide (6T6BA) (IC50=11.0 nmol·L-1, electrophorus).
[1] Hu, M.; Li, J.; Yao, A. Q. Org. Lett. 2008, 10(24), 5529.
[2] Ankati, H.; Biehl, E. Tetrahedron Lett. 2009, 50, 4677.
[3] Zhang, F.; Moses, J. E. Org. Lett. 2009, 11(7), 1587.
[4] Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radi?, Z.; Carlier P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41(6), 1053.
[5] Manetsch, R.; Krasinski, A.; Radic, Z.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2004, 126, 12809.
[6] Krasinski, A.; Radic, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2005, 127, 6686.
[7] Bourne, Y.; Kolb, H. C.; Radi?, Z.; Sharpless, K. B.; Taylor, P.; Marchot, P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1449.
[8] Muñoz-Ruiz, P.; Rubio, L.; García-Palomero, E.; Dorronsoro, I.; Monte-Millán, M.; Valenzuela, R.; Usán, P.; Austria, C.; Bartolini, M.; Andrisano, V.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Medina, M.; Martínez, A. J. Med. Chem. 2005, 48, 7223.
[9] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[10] Lourëat, F.; Bougrin, K.; Loupy, A.; Ochoa de Retana, A. M.; Pagalday, J.; Palacios, F. Heterocycles 1998, 48, 161.
[11] Katritzky, A. R.; Singh, S. K. J. Org. Chem. 2002, 67, 9077
[12] Balducci, E.; Bellucci, L.; Petricci, E.; Taddei, M.; Tafi, A. J. Org. Chem. 2009, 74, 1314.
[13] Guo, Y.-B.; Liu, H.-B.; Xu, M. Chin. J. Synth. Chem. 2011, 19(3), 299 (in Chinese). (郭永彪, 刘海波, 许明, 合成化学, 2011, 19(3), 299.)
[14] Ellman, G. L.; Courtney, K. D.; Andres, V. J.; Featherstone, R. M. Biochem. Pharm. 1961, 7, 88.