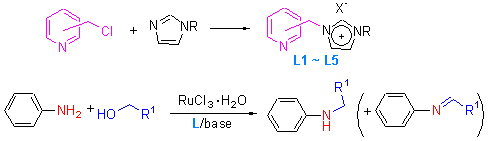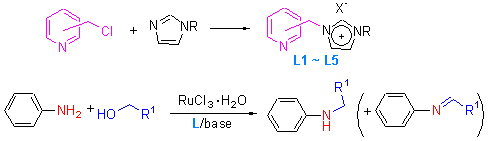[1] Lawrence, S. A. Amines: Synthesis, Properties and Applications, Cambridge University, Cambridge, 2004, p. 207.
[2] Singh, C. B.; Kavala, V.; Samal, A. K.; Patel, B. K. Eur. J. Org. Chem. 2007, 8, 1369.
[3] Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2008, 47, 3096.
[4] Mizuta, T.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 2005, 70, 219.
[5] Ahmed, M.; Buch, C.; Routaboul, L.; Jackstell, R.; Klein, H.; Spannenberg, A.; Beller, M. Chem. Eur. J. 2007, 13, 1594.
[6] Reznichenko, A. L.; Nguyen, H. N.; Hultzsch, K. C. Angew. Chem., Int. Ed. 2010, 49, 8984.
[7] Hultzsch, K. C.; Gribkov, D. V.; Hampel, F. J. Organomet. Chem. 2005, 690, 4441.
[8] (a) Naota, T.; Takaya, H.; Murahashi, S.-I. Chem. Rev. 1998, 98, 2599. (b) Guillena, G.; Ramon, D. J.; Yus, M. Chem. Rev. 2010, 110, 1611. (c) Dobereiner, G.; Crabtree, R. H. Chem. Rev. 2010, 110, 681.
[9] Li, L.-T.; Ma, S.-M. Chin. J. Org. Chem. 2001, 21, 75 (in Chinese). (李林涛, 麻生明, 有机化学, 2001, 21, 75.)
[10] Li, J.-Y.; Peng, J.-J.; Li, X.-N.; Ma, L.; Bai, Y.; Zhang, G.-D.; Lai, G.-Q. Chin. J. Org. Chem. 2010, 30, 1468 (in Chinese). (厉嘉云, 彭家建, 李小年, 马磊, 白赢, 张国栋, 来国桥, 有机化学, 2010, 30, 1468.)
[11] Shi, J.-C.; Cao, J.-J.; Zheng, Y.; Jia, L. Chin. J. Org. Chem. 2007, 27, 666 (in Chinese). (施继成, 曹新华, 郑瑛, 贾莉, 有机化学, 2007, 27, 666.)
[12] Herrmann, W. A.; Kocher, C. Angew. Chem., Int. Ed. Engl. 1997, 36, 2162.
[13] (a) Arduengo, A. J. Acc. Chem. Res. 1999, 32, 913. (b) Peris, E.; Crabtree, R. H. Coord. Chem. Rev. 2004, 248, 2239. (c) Crudden, C. M.; Allan, D. P. Coord. Chem. Rev. 2004, 248, 2247.
[14] James, B. R.; Ochiai, E.; Rempel, G. L. Inorg. Nucl. Chem. Lett. 1971, 7, 781.
[15] Magill, A. M.; McGuinness, D. S.; Cavell, K. J.; Britovsek, G. J. P.; Gibson, V. C.; White, A. J. P.; Williams, D. J.; White, A. H.; Skelton, B. W. J. Organomet. Chem. 2001, 617, 546.
[16] Chiu, P.-L.; Lai, C.-L.; Chang, C.-F.; Hu, C.-H.; Lee, H.-M. Organometallics 2005, 24, 6169.
[17] Wang, X.; Liu, S.; Weng, L. H.; Jin, G. X. J. Organomet. Chem. 2005, 690, 2934.
[18] Watanabe, Y.; Tsuji, Y.; Ohsugi, Y. Tetrahedron Lett. 1981, 22, 2667.
[19] Tsuji, Y.; Kotachi, S.; Huh, K. T.; Watanabe, Y. J. Org. Chem. 1990, 55, 580.