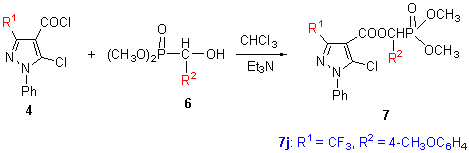
通过1-苯基-3-三氟甲基-5-氯吡唑-4-甲酰氯(4)与α-羟基芳甲基膦酸酯(6)的缩合反应, 合成了14种标题化合物7a~7n. 所有化合物的结构均经1H NMR, IR和元素分析进行了表征, 并且采用X射线单晶衍射分析方法进一步测定了化合物7j的结构. 初步的生物活性测试表明, 部分标题化合物具有一定的杀菌和除草活性.
梁英
,
贺红武
,
何海峰
,
杨自文
. α-(1-苯基-3-三氟甲基-5-氯吡唑-4-甲酰氧基)-α-芳甲基膦酸酯的合成、晶体结构与生物活性[J]. 有机化学, 2012
, 32(08)
: 1513
-1518
.
DOI: 10.6023/cjoc1201041
Fourteen novel α-(substituted pyrazol-4-carbonyloxy)-α-arylmethyl phosphonates 7a~7n were synthesized from the intermediates 5-chloro-3-trifluoromethyl-1-phenylpyrazol-4-carbonyl chloride (4) and α-hydroxyl-α-arylmethyl phos- phonates (6). All compounds synthesized were identified by 1H NMR, IR spectra and elemental analysis, and the structure of 7j was further determined by X-ray single crystal diffraction method. The preliminary bioassay indicated that some compounds showed moderate fungicidal and herbicidal activities.
[1] Kluger, R.; Pike, D. C. J. Am. Chem. Soc. 1977, 99, 4504.
[2] Baillie, A. C.; Wright, K.; Wright, B. J.; Earnshaw, C. G. Pestic. Biochem. Physiol. 1988, 30, 103.
[3] Eto, M. Biosci. Biotechnol. Biochem. 1997, 61, 1.
[4] He, H. W.; Liu, Z. J. Chin. J. Org. Chem. 2001, 21, 878 (in Chinese).(贺红武, 刘钊杰, 有机化学, 2001, 21, 878.)
[5] Chen, T.; Shen, P.; Li, Y. J.; He, H. W. J. Fluorine Chem. 2006, 127, 291.
[6] Chen, T.; Shen, P.; Li, Y. J.; He, H. W. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 2135.
[7] He, H. W.; Chen, T.; Li, Y. J. J. Pestic. Sci. 2007, 32, 42.
[8] Wang, T.; He, H. W.; Miao, F. M. Chin. J. Org. Chem. 2009, 29, 1152 (in Chinese).(王涛, 贺红武, 缪方明, 有机化学, 2009, 29, 1152.)
[9] Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H. W.; Wan, J. J. Agric. Food Chem. 2007, 55, 1871.
[10] He, H. W.; Yuan, J. L.; Peng, H.; Chen, T.; Shen, P.; Wan, S. Q.; Li, Y. J.; Tan, H. L.; He, Y. H.; He, J. B.; Li, Y. J. Agric. Food Chem. 2011, 59, 4801.
[11] Mohareb, R. M.; Azia, S. I.; Abdel-Sayed, N. I. Synth. Commun. 1993, 58, 947.
[12] Boullet, F. T.; Foucaud, A. Synthesis 1982, 916.
[13] Evans, D. A.; Hurst, K. M.; Tabaks, J. M. J. Am. Chem. Soc. 1978, 100, 3467.
[14] Wang, T.; He, H. W. Phosphorus, Sulfur Silicon Relat. Elem. 2004, 179, 2081.
[15] Li, G. H.; Yang, H. Chin. J. Org. Chem. 2008, 28, 1918 (in Chinese).(李国华, 杨红, 有机化学, 2008, 28, 1918.)