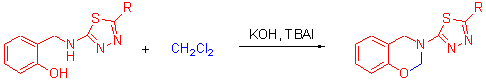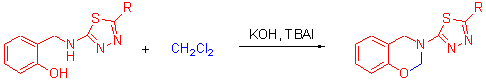[1] Mireya, E. R.; Carrajal, M.A.; Rincon, J. M. Rev. Colomb. Cienc. Quim.-Farm., 1980, 3, 63.
[2] Gomez, P. G.; Pabon, H. P.; Carvajal, M. A.; Rincon, J. M. Rev. Colomb. Cienc. Quim.-Farm. 1985, 8, 15.
[3] Waisser, K.; Gregor, K.; Kubicova, L.; Klimesova, V.; Kunes, J.; Machacek, M.; Kaustova, J. Eur. J. Med. Chem. 2000, 35, 733.
[4] Waisser, K.; Gregor, K.; Dostal, H.; Kunes, J.; Kubicova, L.; Klimesova, V.; Kaustova, J. Il Farmaco, 2001, 56, 803.

[5] Mathiew, B. P.; Kumar. A.; Sharma, S.; Shula, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502.
[6] Chylinska, J. B.; Urbanski, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484.

[7] Bouaziz, Z.; Riondel, J.; Mey, A.; Berlion, M.; Villard, J.; Filliond, H. Eur. J. Med. Chem. 1991, 26, 469.
[8] Benameur, L.; Bouaziz, Z.; Nebois, P.; Bartoli, M-H.; Boitard, M.; Fillion, H. Chem. Pharm. Bull. 1996, 44, 605.

[9] Wang, S.; Li, Y.; You, Q.; Liu, Y.; Lu, A. Bioorg. Med. Chem. Lett., 2008, 18, 4095.

[10] Pasternak, A.; Goble, S. D.; Struthers, M.; Vicario, P. P.; Ayala, J. M.; Salvo, J. D.; Kilburn, R.; Wisniewski, T.; DeMartino. J. A.; Mills, S. G.; Yang,L. ACS Med. Chem. Lett., 2010, 1, 14.

[11] Eva Petrlikova, E.; Waisser, K.; Divisova, H.; Husakova, P.; Vrabcova, P.; Kunes, J.; Kolar, K.; Stolarikova, J. Bioorg. Med. Chem. 2010, 18, 8178.

[12] Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. J. Heterocyclic Chem., 2011, 48, 255.

[13] Huang, W.; Zhao, P-L.; Liu, C-L.; Chen, Q.; Liu, Z-M.; Yang, G-F. J. Agric. Food. Chem. 2007, 55, 3004.

[14] Cao, S.; Wei, N.; Zhao, C.; Li, L.; Huang, Q. Qian, X. J. Agric. Food. Chem. 2005, 53, 3120.

[15] Wang, T.; Bing, G.; Zhang, X.; Qin, Z.; Yu, H.; Qin, X.; Dai, H.; Fang, J. Chin. J. Org. Chem. 2009, 29, 1287 (in Chinese).(王婷婷,邴贵方,张欣,秦振芳,于海波,秦雪,戴红,方建新,有机化学,2009,29,1287.)
[16] Yang, C.; Yang, S.; Song, B.; Hu, D.; Chen, H.; Xue, W.; Jin, L.; Wu, J.; Xu, W.; Bai, S. Chin. J. Org. Chem. 2010, 30, 1327 (in Chinese).(杨超,杨松,宋宝安,胡德禹,陈红军,薛伟,金林红,吴剑,徐伟明,柏松,有机化学,2010,30,1327.)
[17] Burke, W. J. J. Am. Chem. Soc. 1949, 71, 609.

[18] Burke, W. J.; Ec, K. C.; Murdock, G. J. Am. Chem. Soc. 1954, 76, 1677.

[19] Rivera, A.; Ospina, E.; Sanchez, A.; Joseph-Nathan, P. Heterocycles, 1986, 24, 2507.

[20] McDonagh, A. F.; Smith, H. E. J. Org. Chem. 1968, 33, 1.

[21] Neuvonen, K.; Pihlaja, K. J. Chem. Soc. Perkin. Trans.II, 1988, 461.
[22] Colin, J. L.; Loubinoux, B. Tetrahedron Lett. 1982, 23, 4245
[23] Campi, E. M.; Jackson, W. R.; McCubbin, Q. J.; Trnacek, A. E. J. Chem. Soc. Chem. Commun. 1994, 2763.
[24] Starks, C. M.; Liotta, C. L.; Halpern, M. Phase-Transfer Catalysis; Chapman & Hall: New York, 1994.
[25] Sasson, Y., Neumann, R., Eds. Handbook of Phase-Transfer Catalysis; Blackie Academic & Professional: London, 1997.
[26] Palmieri, G. Eur. J. Org. Chem. 1999, 805.
[27] Tang, Z.; Chang, S.; Yan, L.; Zhang, C. Liu, H. Chin. J. Applied Chem. 2012 (in Chinese, in press).(唐子龙,常书红,颜林,张超逸,刘汉文,应用化学,2012 (已排版)).
[28] Liu, A.; Ou, X.; Huang, M.; Wang, X.; Liu, X.; Wang, Y.; Chen, C.; Yao, J. Pest Manage Sci, 2005, 61, 166.