

新型萘基修饰四硫富瓦烯衍生物的合成及性质研究
收稿日期: 2011-06-26
修回日期: 2011-12-25
网络出版日期: 2012-01-06
基金资助
国家自然科学基金(Nos. 20872058, 21172105)资助项目.
Synthesis and Properties of A Novel Naphthol-Substituted Tetrathiafulvalene Derivative
Received date: 2011-06-26
Revised date: 2011-12-25
Online published: 2012-01-06
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20872058, 21172105).
赵邦屯 , 刘连委 , 李晓川 , 渠桂荣 . 新型萘基修饰四硫富瓦烯衍生物的合成及性质研究[J]. 有机化学, 2012 , 32(05) : 953 -956 . DOI: 10.6023/cjoc1107262
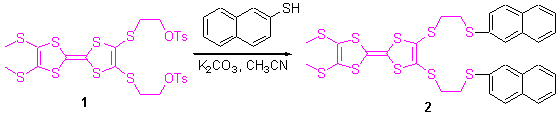
Key words: tetrathiafulvalene; naphthalene thiol; ions recognition








/
| 〈 |
|
〉 |