

微波辐射下离子液体催化一锅三组分反应合成 3-{1-[2-(4-芳基噻唑-2-基)亚肼基]乙基}香豆素
收稿日期: 2011-02-26
修回日期: 2012-06-01
网络出版日期: 2012-05-12
基金资助
河北省科技厅(No.11276434)和保定市科技局(No.10ZF104)资助项目
Microwave-Assisted Ionic Liquid Catalyzed One-Pot Three-Component Synthesis of 3-{1-[2-(4-Arylthiazol-2-yl)-hydrazono]ethyl}-2H-chromen-2-one
Received date: 2011-02-26
Revised date: 2012-06-01
Online published: 2012-05-12
Supported by
Project supported by the Science and Technology Department of Hebei Province (No.11276434) and the Science and Technology Bureau of Baoding City (No.10ZF104)
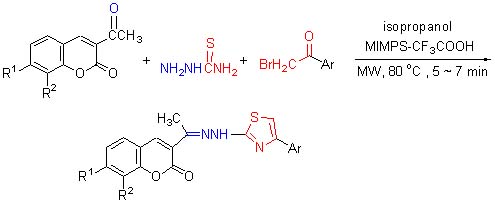
关键词: 酸性离子液体; 3-{1-[2-(4-芳基噻唑-2-基)亚肼基]乙基}香豆素; 微波辐射; 合成
张冬暖 , 李记太 , 刘卉闵 , 宋亚丽 , 张英群 , 袁明月 . 微波辐射下离子液体催化一锅三组分反应合成 3-{1-[2-(4-芳基噻唑-2-基)亚肼基]乙基}香豆素[J]. 有机化学, 2012 , 32(9) : 1732 -1735 . DOI: 10.6023/cjoc1202261


/
| 〈 |
|
〉 |