

3-(2-氯-4-三氟甲基)苯氧基取代苯甲酰腙衍生物的合成与生物活性
收稿日期: 2012-04-20
修回日期: 2012-05-24
网络出版日期: 2012-06-05
基金资助
国家重点基础研究发展规划(973计划) (No. 2010CB126100)、国家自然科学基金(Nos. 20372023, 20772042)资助项目.
Synthesis and Pesticidal Activity of 3-(2-Chloro-4-trifluoromethyl)phenoxy Benzoylhydrazones
Received date: 2012-04-20
Revised date: 2012-05-24
Online published: 2012-06-05
Supported by
Project supported by the National Basic Research Program of China (No. 2010CB126100), and the National Natural Science Foundation of China (Nos. 20372023, 20772042).
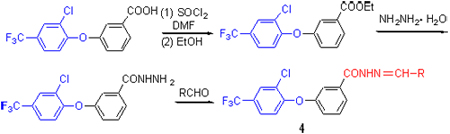
选择3-(2-氯-4-三氟甲基苯氧基)苯甲酸为起始原料, 经酰氯化, 再与乙醇反应成酯, 然后和水合肼反应, 最后与芳香醛缩合, 合成了10个未见文献报道的3-(2-氯-4-三氟甲基苯氧基)苯甲酰腙4. 通过IR, 1H NMR, EI-MS, 元素分析等方法对所合成的化合物进行了结构表征. 代表化合物4-氯苯亚甲基-3-(2-氯-4-三氟甲基苯氧基)苯甲酰腙(4f)经单晶X衍射证实了结构. 并初步测定了所合成化合物的杀菌和除草活性. 结果表明: 部分测试化合物在50 mg/L浓度下对水稻纹枯菌和黄瓜灰霉菌具有优良的抑制效果, 在100 mg/L浓度下对油菜和稗草的根显示一定的抑制效果.
刘建超 , 崔泽平 , 贺红武 . 3-(2-氯-4-三氟甲基)苯氧基取代苯甲酰腙衍生物的合成与生物活性[J]. 有机化学, 2012 , 32(10) : 1925 -1929 . DOI: 10.6023/cjoc201204022
A series of new substituted benzaldehyde (or 2-furaldehyde) 3-(2-chloro-4-trifluoromethyl)phenoxy benzoylhy- drazones 4 have been designed and synthesized by the reactions of substituted aldehydes with intermediate 3 in 64%~89% yields. The structures of compounds 4 have been confirmed by 1H NMR, IR, EI-MS and elemental analyses. The structure of 4-chloro-benzaldehyde 3-(2-chloro-4-trifluoromethyl)phenoxy benzoylhydrazone (4f) has been determined by X-ray single crystal diffraction. The results of preliminary bioassay indicated that some compounds possess good fungicidal activities against Rhizoctonia solani and Botrytis cinereapers at a dosage of 50 mg/L and moderate herbicidal activity against the roots of rape and barnyard grass at 100 mg/L.

Key words: benzaldehyde; benzoylhydrazone; fungicidal activity; herbicidal activity
[1] Singh, V. P.; Katiyar, A. Pestic. Biochem. Physiol. 2008, 92, 8.
[2] Ni, Z.; Xue, S.; Wang, J.; Meng, W. Chin. J. Org. Chem. 2011, 32, 222 (in Chinese).
(倪振杰, 薛思佳, 王静, 孟雯, 有机化学, 2011, 32, 222.)
[3] Ling, A. L.; Hong, Y.; Gonzalez, J.; Gregor, V.; Polinsky, A.; Kuki, A.; Shi, S.; Teston, K.; Murphy, D.; Porter, J.; Kiel, D.; Lakis, J.; Anderes, K.; May, J.; Knudsen, L. B.; Jesper. L. J. Med. Chem. 2001, 44, 3141.
[4] Ling, A.; Plewe, M.; Gonzalez, J.; Madsen, P.; Sams, C. K.; Lau, J.; Gregor, V.; Murphy, D.; Teston, K.; Kuki, A.; Shi, S.; Truesdale, L.; Kiel, D.; May, J.; Lakis, J.; Anderes, K.; Iatsimirskaia, E.; Sidelmann, U. G.; Knudsen, L. B.; Btand, C. L.; Polinsky, A. Bioorg. Med. Chem. Lett. 2002, 12, 663.
[5] Bernhardt, P. V.; Mattsson, J.; Richardson, D. R. Inorg. Chem. 2006, 45, 752.
[6] Kalinowski, D. S.; Sharpe, P. C.; Bernhardt, P. V.; Richardson, D. R. J. Med. Chem. 2008, 51, 331.
[7] Wang, M. H.; Yang, C. L.; Jiang, M. G. World Pestic. 2002, 24, 13 (in Chinese).
(王鸣华, 杨春龙, 蒋木庚, 世界农药, 2002, 24, 13.)
[8] Song, X.; Wang, S.; Tan, X.; Wang, Z.; Wang, Y. Chin. J. Org. Chem. 2007, 27, 72 (in Chinese).
(宋新建, 王胜, 谭小红, 王子云, 汪焱钢, 有机化学, 2007, 27, 72.)
[9] Peng, H.; He, H. W. Chin. J. Org. Chem. 2007, 27, 502 (in Chinese).
(彭浩, 贺红武, 有机化学, 2007, 27, 502.)
[10] Chen, T.; Shen, P.; Li, Y. J.; He, H. W. J. Fluorine Chem. 2006, 127, 291.
[11] Cui, Z.; Li, Y.; Ling, Y.; Huang, J.; Cui, J.; Wang, R.; Yang, X. Eur. J. Med. Chem. 2010, 45, 5576.
/
| 〈 |
|
〉 |