

含有对氨基苯甲酸和苯磺酰胺结构单元的新型分子及其抗糖尿病活性
收稿日期: 2012-04-13
修回日期: 2012-05-31
网络出版日期: 2012-10-27
基金资助
重庆市科技攻关计划(Nos. 2011AB5001, 2011AC1053, 2011AC5107)资助项目.
Synthesis and Antidiabetic Activity of Novel Molecules Containing p-Aminobenzoic Acid and Benzenesulfonamide Moiety
Received date: 2012-04-13
Revised date: 2012-05-31
Online published: 2012-10-27
Supported by
Project supported by the Scientific and Technological Project in Chongqing City (Nos. 2011AB5001, 2011AC1053, 2011AC5107).
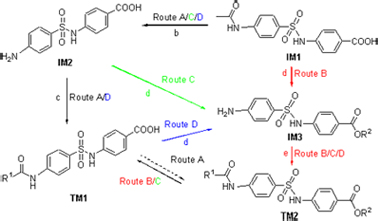
基于本实验室前期发现的高活性分子的结构特征, 作者设计了含有对氨基苯甲酸和苯磺酰胺结构单元的新型分子. 通过4条合成路线的探索, 发现了中间体IM1~IM3及目标分子TM1和TM2的简捷合成路线和实用合成方法; 采用本实验室前期建立的合成方法, 顺利得到设计的26个化合物, 合成方法简便, 反应条件温和, 收率为64%~95%. 21个新化合物通过1H NMR,13C NMR和HRMS进行结构表征. 目前的体外抗糖尿病活性结果显示, 所得26个分子的过氧化物酶体增殖物激活受体(PPAR)的激动活性较弱. 本研究进一步证实了醇/二氯亚砜体系脱除脂肪酰芳胺酰基的能力, 有助于含有对氨基苯甲酸和苯磺酰胺结构单元分子的进一步结构优化.
杨龙 , 晏菊芳 , 范莉 , 陈欣 , 上官瑞燕 , 汪林发 , 杨大成 . 含有对氨基苯甲酸和苯磺酰胺结构单元的新型分子及其抗糖尿病活性[J]. 有机化学, 2012 , 32(10) : 1908 -1918 . DOI: 10.6023/cjoc201204016
A new class of target molecules containing p-aminobenzoic acid and benzenesulfonamide moiety is reported, based on the structural features of a series of analogues with strong biological activities previously synthesized by the group. By taking 4 discrete synthetic routes, the practical procedures and facile preparative routes for both the intermediates of IM1~IM3 and the target molecules of TM1 and TM2 were established. A total of 26 designed compounds were synthesized smoothly using the established synthetic approaches under mild reaction conditions, with low cost and high yields (64%~95%). The chemical structures of 21 new compounds were confirmed by 1H NMR, 13C NMR and HRMS techniques. The bioassay test demonstrates weak antidiabetic activity for all the target molecules. This study has further expanded the application of alcohol/SOCl2 system in the deacylation of N-arylacetamides and chloro-N-arylacetamides as well as esterification of carboxy group concomitantly, which is supportive to the structure optimization of novel molecules containing p-aminobenzoic acid and benzenesulfonamide moiety.

[1] Xu, Y. P.; Etgen, G. J.; Broderick, C. L.; Canada, E.; Gonzalez, I.; Lamar, J.; Montrose-Rafizadeh, C.; Oldham, B. A.; Osborne, J. J., Xie, C. Y.; Shi, Q.; Winneroski, L. L.; York, J.; Yumibe, N.; Zink, R.; Mantlo, N. J. Med. Chem. 2006, 49, 5649.
[2] Yoshida, K.; Hishida, A.; Iida, O.; Hosokawa, K.; Kawabata, J. J Agric. Food Chem. 2008, 56, 4367.
[3] Mohler, M. L.; He Y. L.; Wu Z. Z.; Hwang, D. J.; Miller, D. D. Med. Res. Rev. 2009, 29, 125.
[4] Kim, S. J.; Jung, M. H.; Yoo, K. H.; Cho, J. H.; Oh, C. H. Bioorg. Med. Chem. Lett. 2008, 18, 5815.
[5] Temperini, C.; Cecchi, A.; Scozzafava, A.; Supuran, C. T. Bioorg. Med. Chem. Lett. 2008, 18, 2567.
[6] Rothschild, C. M.; Hines, M. T.; Breuhaus, B.; Gay, J.; Sellon, D. C. J. Vet. Intern. Med. 2004, 18, 370.
[7] Tanaka, H.; Ohshima, N.; Hidaka, H. Mol. Pharmacol. 1999, 55, 356.
[8] Yang, H. T.; Endoh, M. Eur. J. Pharmacol. 1996, 312, 281.
[9] Hao, G. F.; Yang, G. F. Chin. J. Org. Chem. 2008, 28, 1545 (in Chinese).
(郝格非, 杨光富, 有机化学, 2008, 28, 1545.)
[10] Seri, K.; Kazuko, S.; Katsumi, K.; Yorishige, I.; Hiroyuki, A. Eur. J. Pharmacol. 2000, 389, 253.
[11] Basset, G. J. C.; Quinlivan, E. P.; Ravanel, S.; Rébeillé, F.; Nichols, B. P.; Shinozaki, K.; Seki, M.; Adams-Phillips, L. C.; Giovannoni, J. J.; Gregory III, J. F.; Hanson, A. D. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1496.
[12] Pawelczak, K.; Jones, T. R.; Kempny, M.; Jackman, A. L.; Newell, D. R.; Krzyzanowski, L.; Rzeszotarska, B. J. Med. Chem. 1989, 32, 160.
[13] Lahue, B. R.; Wan, Z. K.; Snyder, J. K. J. Org. Chem. 2003, 68, 4345.
[14] Ohkanda, J.; Buckner, F. S.; Lockman, J. W.; Yokoyama, K.; Carrico, D.; Eastman, R.; Luca-Fradley, K. D.; Davies, W.; Croft, S. L.; Voorhis, W. C. V.; Gelb, M. H.; Sebti, S. M.; Hamilton, A. D. J. Med. Chem. 2004, 47, 432.
[15] Sun, J.; Yang, Y. S.; Li, W.; Zhang, Y. B.; Wang, X. L.; Tang, J. F.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2011, 21, 6116.
[16] Prabhu, P. P.; Shastry, C. S.; Pande, S.; Pai, A. J. Pharm. Res. 2011, 4, 2209.
[17] Qiu, J. Y.; Xu, B.; Huang, Z. M.; Pan, W. D.; Cao, P. X.; Liu, C. X.; Hao, X. J.; Song, B. A.; Liang, G. Y. Bioorg. Med. Chem. 2011, 19, 5352.
[18] Chen, X. F.; Wu, Y. B.; Jin, J.; Wang, R. Z.; Wang, C.; Liu, J. Acta Pharm. Sin. 2010, 45, 263 (in Chinese).
(陈晓芳, 武燕彬, 金洁, 王瑞贞, 王翀, 刘浚, 药学学报, 2010, 45, 263.)
[19] Mulongo, G.; Mbabazi, J.; Odongkara, B.; Twinomuhwezi, H.; Mpango, G. B. Res. J. Chem. Sci. 2011, 1, 102.
[20] Pfefferkorn, J. A.; Song, Y. T.; Sun, K. L.; Miller, S. R.; Trivedi, B. K.; Choi, C.; Sorenson, R. J.; Bratton, L. D.; Unangst, P. C.; Larsen, S. D.; Poel, T. J.; Cheng, X. M.; Lee, C.; Erasga, N.; Auerbach, B.; Askew, V.; Dillon, L.; Hanselman, J. C.; Lin, Z. W.; Lu, G.; Robertson, A.; Olsen, K.; Mertz, T.; Sekerke, C.; Pavlovsky, A.; Harris, M. S.; Bainbridge, G.; Caspers, N.; Chen, H. F.; Eberstadt, M. Bioorg. Med. Chem. Lett. 2007, 17, 4538.
[21] Dhananjeyan, M. R.; Trendel, J. A.; Bykowski, C.; Sarver, J. G.; Ando, H.; Erhardt, P. W. J. Chromatogr., B 2008, 867, 247.
[22] Yang, D. C.; Yan, J. F.; Xu, J.; Ye, F.; Zhou, Z. W.; Zhang, W. Y.; Fan, L.; Chen, X. Acta Pharm. Sin. 2010, 45, 66 (in Chinese).
(杨大成, 晏菊芳, 许荩, 叶飞, 周祖文, 张蔚瑜, 范莉, 陈欣, 药学学报, 2010, 45, 66.)
[23] Zhang, K.; Yan, J. F.; Tang, X. M.; Liu, H. P.; Fan, L.; Zhou, G. M.; Yang, D. C. Acta Pharm. Sin. 2011, 46, 412 (in Chinese).
(张坤, 晏菊芳, 唐雪梅, 刘红萍, 范莉, 周光明, 杨大成, 药学学报, 2011, 46, 412.)
[24] Tang, X. M.; Yan, J. F.; Zhang, Y. X.; Zhang, W. Y.; Su, X. Y.; Chen, X.; Zhou, Z. W.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 1790 (in Chinese).
(唐雪梅, 晏菊芳, 张映霞, 张蔚瑜, 苏小燕, 陈欣, 周祖文, 杨大成, 有机化学, 2009, 29, 1790.)
[25] Song, X. L.; Yan, J. F.; Fan, L.; Chen, X.; Xu, J.; Zhou, Z. W.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 606 (in Chinese).
(宋小礼, 晏菊芳, 范莉, 陈欣, 许荩, 周祖文, 杨大成, 有机化学, 2009, 29, 606.)
[26] Tang, X. M.; Fan, L.; Yu, H. X.; Liao, Y. H.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 595 (in Chinese).
(唐雪梅, 范莉, 于红霞, 廖玉华, 杨大成, 有机化学, 2009, 29, 595.)
[27] Novacek, A.; Sedlackova, V.; Korner, J.; Danek, J. CS 266792, 1990 [Chem. Abstr. 1991, 114, 246957].
[28] Rajagopalan, S. Proc.-Indian Acad. Sci., Sect. A 1943, 18A, 108.
[29] Yang, D. C.; Wang, L. F.; Fan, L.; Wang, G. B.; Shang-Guan, R. Y.; Sun, J.; Li, C. Z.; Yang, L. CN 102153481, 2011 [Chem. Abstr. 2011, 155, 352231].
[30] Wang, G. B.; Wang, L. F.; Li, C. Z.; Sun, J.; Zhou, G. M.; Yang, D. C. Res. Chem. Intermed. 2012, 38, 77.
[31] Meshram, G. A.; Patil, V. D. Tetrahedron Lett. 2009, 50, 1117.
[32] Bahrami, K.; Khodaei, M. M.; Soheilizad, M. J. Org. Chem. 2009, 74, 9287.
[33] Palakurthy, N. B.; Mandal, B. Tetrahedron Lett. 2011, 52, 7132.
[34] Yu, X. F.; Lu, X. Y. Adv. Synth. Catal. 2011, 353, 2805.
[35] Luo, X. X.; Bai, H.; Xue, X. Y.; Hou, Z.; Zhou, Y.; Sang, G. J.; Meng, J. R. CN 101857562, 2010 [Chem. Abstr. 2010, 153, 554743].
[36] Pastor-Navarro, N.; García-Bover, C.; Maquieira, á.; Puchades, R. Anal. Bioanal. Chem. 2004, 379, 1088.
[37] Mirza, S. M.; Mustafa, G.; Khan, I. U.; Zia-Ur-Rehman, M.; Shafiq, M. Acta Crystallogr., Sect. E 2010, E67, o25.
[38] Chitranshi, P.; Xue, L. Bioorg. Med. Chem. Lett. 2011, 21, 6357.
[39] Motoshima, K.; Ishikawa, M.; Hashimoto, Y.; Sugita, K. Bioorg. Med. Chem. 2011, 19, 3156.
[40] Ismail, N. S. M.; Hattori, M. Bioorg. Med. Chem. 2011, 19, 374.
[41] Hutchinson, J. H.; Li, Y. W.; Arruda, J. M.; Baccei, C.; Bain, G.; Chapman, C.; Correa, L.; Darlington, J.; King, C. D.; Lee, C.; Lorrain, D.; Prodanovich, P.; Rong, H. J.; Santini, A.; Stock, N.; Prasit, P.; Evans, J. F. J. Med. Chem. 2009, 52, 5803.
[42] Suryadevara, P. K.; Olepu, S.; Lockman, J. W.; Ohkanda, J.; Karimi, M.; Verlinde, C. L. M. J.; Kraus, J. M.; Schoepe, J.; Voorhis, W. C. V.; Hamilton, A. D.; Buckner, F. S.; Gelb, M. H. J. Med. Chem. 2009, 52, 3703.
[43] Takaya, J.; Miyashita, Y.; Kusama, H.; Iwasawa, N. Tetrahedron 2011, 67, 4455.
[44] Rewcastle, G. W.; Gamage, S. A.; Flanagan, J. U.; Frederick, R.; Denny, W. A.; Baguley, B. C.; Kestell, P.; Singh, R.; Kendall, J. D.; Marshall, E. S.; Lill, C. L.; Lee, W. J.; Kolekar, S.; Buchanan, C. M.; Jamieson, S. M. F.; Shepherd, P. R. J. Med. Chem. 2011, 54, 7105.
[45] Brenna, E.; Fuganti, C.; Gatti, F. G.; Parmeggiani, F. Tetrahedron: Asymmetry 2009, 20, 2594.
[46] Sliman, F.; Desmaele, D. Synthesis 2010, 619.
[47] Shi, G. Q.; Dropinski, J. F.; McKeever, B. M.; Xu, S. H.; Becker, J. W.; Berger, J. P.; MacNaul, K. L.; Elbrecht, A.; Zhou, G. C.; Doebber, T. W.; Wang, P. R.; Chao, Y. S.; Forrest, M.; Heck, J. V.; Moller, D. E.; Jones, A. B. J. Med. Chem. 2005, 48, 4457.
[48] Kumar, A.; Maurya, R. A.; Sharma, S.; Ahmad, P.; Singh, A. B.; Tamrakar, A. K.; Srivastava, A. K. Bioorg. Med. Chem. 2009, 17, 5285.
[49] Yang, D. C.; Yan, J. F.; Wang, L. F.; Ye, F.; Fan, L.; Mou, X.; Li, T. J.; Liu, H. L.; Chen, W. D.; Xu, J.; Song, X. L. CN 101735286, 2010 [Chem. Abstr. 2010, 153, 116535].
[50] Corradi, V.; Mancini, M.; Santucci, M. A.; Carlomagno, T.; Sanfelice, D.; Mori, M.; Vignaroli, G.; Falchi, F.; Manetti, F.; Radi, M.; Botta, M. Bioorg. Med. Chem. Lett. 2011, 21, 6867.
[51] Yao, Z. Y.; Yang, D. C.; Fan, L. Chin. J. Med. Chem. 2003, 13, 16 (in Chinese).
(姚志勇, 杨大成, 范莉, 中国药物化学杂志, 2003, 13, 16.)
[52] Gupta, P.; Paul, S. Green Chem. 2011, 13, 2365.
[53] Rajput, A. P.; Gore, R. P. Der Pharma Chem. 2011, 3, 409.
[54] Balaskar, R. S.; Gavade, S. N.; Mane, M. S.; Shingare, M. S.; Mane, D. V. Green Chem. Lett. Rev. 2011, 4, 91.
[55] Chou, C. T.; Yellol, G. S.; Chang, W. J.; Sun, M. L.; Sun, C. M. Tetrahedron 2011, 67, 2110.
[56] Berger, J.; Moller, D. E. Annu. Rev. Med. 2002, 53, 409. Krentz, A. J.; Bailey, C. J. Drugs 2005, 65, 385.
/
| 〈 |
|
〉 |