

过渡金属催化下二醋酸碘苯-四正丁基溴化铵与苯乙烯衍生物选择性加成反应的研究
收稿日期: 2012-05-17
修回日期: 2012-06-05
网络出版日期: 2012-06-11
基金资助
江苏省高校优势学科、浙江省重中之重学科开放基金(No. 100061200138)、江苏省自然科学基金(No. BK2010321)、江苏省高校自然科学基金(No. 09KJB150014)及江苏省重点实验室开放基金(No. K100027)资助项目.
An Investigation on the Transition Metal Catalyzed Selective Additions of Styrenes with Diacetoxyiodobenzene (DIB)- Tetrabutylamidebromide (TBAB) System
Received date: 2012-05-17
Revised date: 2012-06-05
Online published: 2012-06-11
Supported by
Project supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Opening Foundation of Zhejiang Provincial Top Key Discipline (No. 100061200138), the Natural Scientific Foundation of Jiangsu Province (No. BK2010321), the University Natural Scientific Foundation of Jiangsu Province (No. 09KJB150014) and the Opening Labortary Fundation of Jiangsu Province (No. K100027).
范磊 , 任玲凤 , 陈天 , 易容 , 俞磊 . 过渡金属催化下二醋酸碘苯-四正丁基溴化铵与苯乙烯衍生物选择性加成反应的研究[J]. 有机化学, 2012 , 32(08) : 1439 -1444 . DOI: 10.6023/cjoc201205023
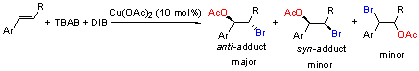
Key words: catalysis; transition metal; regioselecticvity; stereoselecticvity; addition
/
| 〈 |
|
〉 |