

查尔酮及其螺杂环衍生物的合成、晶体结构、抗氧化活性研究
收稿日期: 2012-04-01
修回日期: 2012-06-18
网络出版日期: 2012-07-02
基金资助
浙江省医药卫生科技计划(No. 2012KYA129)、温州市科技局(No. Y20090101)、浙江省中医药科技计划B类(No. 2012ZB102)资助项目.
Synthesis, Crystal Structure, Antioxidant Activity of Chalcones and Its Spiro-heterocyclic Analogues
Received date: 2012-04-01
Revised date: 2012-06-18
Online published: 2012-07-02
Supported by
Project supported by the Technology Foundation for Medical Science of Zhejiang Province (No. 2012KYA129), the Project of Wenzhou Sci & Tech Bureau (No. 20090101), and the Technology Foundation for Chinese Medicine of Zhejiang Province (No. 2012ZB102).
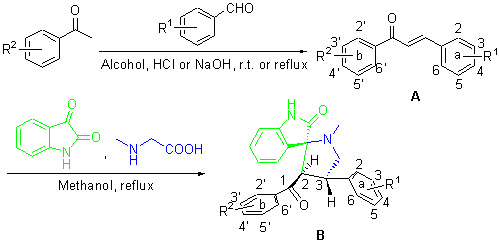
为了合成新结构类型查尔酮衍生物, 发现具有抗氧化活性的查尔酮类化合物, 设计合成了查尔酮A和螺杂环B两种类型, 共21个查尔酮类似物, 结构经ESI-MS, ESI-HRMS和1H NMR确认. 培养出螺杂环B1的单晶, 通过X衍射确证了其为单斜晶系. 其中螺杂环B为新结构类型化合物, 通过1,3-偶极环加成反应, 用不需加催化剂的“一锅煮”方法合成, 该反应具有很好的立体选择性和区域选择性、且环境友好. 用DPPH法测试了所有化合物的抗氧化活性, 筛选出了多个对1,1-二苯基-2-三硝基苯肼(DPPH)自由基具有良好清除率的化合物, a环3,4-OH取代的两类化合物都具有良好的抗氧化活性, 苯环邻位二羟基取代的查尔酮类化合物可能具有很好的抗氧化活性.
吴建章 , 李物兰 , 陈玲姿 , 楚生辉 , 赵承光 , 卫涛 , 杨树林 , 李校堃 . 查尔酮及其螺杂环衍生物的合成、晶体结构、抗氧化活性研究[J]. 有机化学, 2012 , 32(11) : 2141 -2147 . DOI: 10.6023/cjoc201204001
To synthesize new structure type chalcone analogues, and obtain chalcone analogues with good antioxidant activity. Two types of chalcone analogs, chalcones A and spiro-heterocyclic B were designed and synthesized, and the structures of 21 compounds were characterized by 1H NMR, ESI-MS and ESI-HRMS. Single-crystal of spiro-heterocyclic B was cultured, and its single-crystal structure was determined by X-ray diffraction study. The crystal structure of B1 was monoclinic system, space group C2/c, with cell dimensions of a=21.350(3) Å, b=8.6256(10) Å, c=26.161(3) Å. Spiro-heterocyclic B is the new structure type, and obtained by one-pot synthesis which is 1,3-dipolar cycloaddition reaction and no catalyst. The synthesis of spiro-heterocyclic B is not only high regioselectivity and stereoselectivity, but also environmentally friendly. The antioxidant activities in vitro were evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. Many compounds, which can have strong activity of scavenging DPPH free radicals, were screened. In the 2 types of chalcones analogs, the compounds with 3,4-(OH)2 in “a” ring have excellent antioxidant activities. The chalcone analogs with o-dihydroxy in benzene ring maybe have good antioxidant activities.

Key words: chalcones; spiro-heterocyclic; synthesis; DPPH; antioxidant activities; crystal structure
/
| 〈 |
|
〉 |