

含3,4-二氢-2(1H)-喹啉酮结构查尔酮衍生物的合成及蛋白酪氨酸磷酸酯酶1B抑制活性研究
收稿日期: 2012-06-18
修回日期: 2012-07-16
网络出版日期: 2012-07-19
基金资助
国家自然科学基金(Nos. 20962021, 81125023)资助项目.
Synthesis and Protein Tyrosine Phosphatase 1B Inhibitory Activity of Novel Chalcone Derivatives Containing 3,4-Dihydroquinolin-2(1H)-one Moiety
Received date: 2012-06-18
Revised date: 2012-07-16
Online published: 2012-07-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20962021, 81125023).
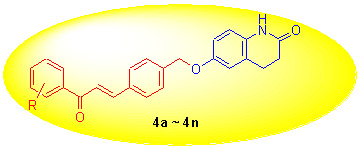
蛋白酪氨酸磷酸酶1B (PTP1B)作为胰岛素和瘦素信号转导通路的负调节因子, 已成为治疗糖尿病和肥胖症的潜在靶标. 为了寻找非磷酸酯类PTP1B抑制剂, 设计、合成了一系列含3,4-二氢-2(1H)-喹啉酮结构的新型查尔酮衍生物, 并对化合物进行了PTP1B抑制活性测定. 结果显示, 所有化合物对PTP1B均显示出较强的抑制活性, 其中化合物(E)-6-{4-[3-(4-氯苯基)-3-氧代-1-丙烯基]苄氧基}-3,4-二氢-2(1H)-喹啉酮(4e)和(E)-6-{4-[3-(3-溴苯基)-3-氧代-1-丙烯基]苄氧基}-3,4-二氢-2(1H)-喹啉酮(4i)活性最佳, IC50分别为(4.64±0.38)和(4.36±0.41) μmol/L.
关键词: 查尔酮; 3,4-二氢-2(1H)-喹啉酮; PTP1B抑制剂
孙良鹏 , 姜哲 , 高立信 , 李英哲 , 孙立云 , 李佳 , 朴虎日 . 含3,4-二氢-2(1H)-喹啉酮结构查尔酮衍生物的合成及蛋白酪氨酸磷酸酯酶1B抑制活性研究[J]. 有机化学, 2012 , 32(11) : 2108 -2114 . DOI: 10.6023/cjoc201206018
Protein tyrosine phosphatase 1B (PTP1B) has recently been identified as new drug target for type II diabetes and obesity due to it is a negative regulator of the insulin and leptin-signaling pathway. In order to find new nonphosphonate-based pTyr mimetics, a series of novel chalcone derivatives bearing the 3,4-dihydroquinolin-2(1H)-one moieties (4a~4n) were designed, synthesized, and evaluated for their PTP1B inhibitory activity. The results demonstrated that all compounds presented potent inhibitory activities against PTP1B, among which compounds 4e and 4i exhibited most potent with IC50 value of (4.64±0.38) and (4.36±0.41) μmol/L, respectively.

Key words: chalcone; 3,4-dihydroquinolin-2(1H)-one; PTP1B inhibitor
[1] (a) Neel, B. G.; Tonks, N. K. Curr. Opin. Cell Biol. 1997, 9, 193.
(b) Hunter, T. Cell 2000, 100, 113.
(c) Tonks, N. K.; Neel, B.G. Curr. Opin. Cell Biol. 2001, 13, 182.
[2] (a) Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A. L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan C. C.; Ramachandran, C.; Gresser, M. J.; Tremblay, M. L.; Kennedy, B. P. Science 1999, 283, 1544.
(b) Klaman, L. D.; Boss, O.; Peroni, O. D.; Kim, J. K.; Martino, J. L.; Zabolotny, J. M.; Moghal, N.; Lubkin, M.; Kim, Y. B.; Sharpe, A. H.; Stricker-Krongrad, A.; Shulman, G. I.; Neel, B. G.; Kahn, B. B. Mol. Cell Biol. 2000, 20, 5479.
[3] Zinker, B. A.; Rondinone, C. M.; Trevillyan, J. M.; Gum, R. J.; Clampit, J. E.; Waring, J. F.; Xie, N.; Wilcox, D.; Jacobson, P.; Frost, L.; Kroeger, P. E.; Reilly, R. M.; Koterski, S.; Opgenorth, T. J.; Ulrich, R. G.; Crosby, S.; Butler, M.; Murray, S. F.; McKay, R. A.; Bhanot, S.; Monica, B. P.; Jirousek, M. R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 11357.
[4] Rondinone, C. M.; Trevillyan, J. M.; Clampit, J. E.; Gum, R. J.; Berg, C.; Kroeger, P.; Frost, L.; Zinker, B. A.; Reilly, R.; Ulrich, R.; Butler, M.; Monia, B. P.; Jirousek, M. R.; Waring, J. F. Diabetes 2002, 51, 2405.
[5] Ramachandran, C.; Kennedy, B. P. Curr. Top Med. Chem. 2003, 3, 749.
[6] Combs, A. P. J. Med. Chem. 2010, 53, 2333.
[7] Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Bioorg. Med. Chem. 2008, 16, 4286.
[8] (a) Chen, Z. H.; Zheng, C. J.; Sun, L. P.; Piao, H. R. Eur. J. Med. Chem. 2010, 45, 5739.
(b) Liu, X. F.; Zheng, C. J.; Sun, L. P.; Liu, X. K.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3469.
[9] Chiaradia, L. D.; dos Santos, R.; Vitor, C. E.; Vieira, A. A.; Leal, P. C.; Nunes, R. J.; Calixto, J. B.; Yunes, R. A. Bioorg. Med. Chem. 2008, 16, 658.
[10] Vogel, S.; Heilmann, J. J. Nat. Prod. 2008, 71, 1237.
[11] Domínguez, J. N.; León, C.; Rodrigues, J.; Gamboa de Domínguez, N.; Gut, J.; Rosenthal, P. J. J. Med. Chem. 2005, 48,3654.
[12] Daskiewicz, J. B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J. M.; Johnson, I. T.; Ndjoko, K.; Hostettmann, K.; Barron, D. J. Med. Chem. 2005, 48, 2790.
[13] Sui, X.; Quan, Y. C.; Chang, Y.; Zhang, R. P.; Xu, Y. F.; Guan, L. P. Med. Chem. Res. 2011, 20, 1.
[14] Chen, R. M.; Hu, L. H.; An, T. Y.; Li, J.; Shen, Q. Bioorg. Med. Chem. Lett. 2002, 12, 3387.
[15] Yoon, G.; Lee, W.; Kim, S. N.; Cheon, S. H. Bioorg. Med. Chem. Lett. 2009, 19, 5155.
[16] Liu, Z.; Lee, W.; Kim, S. N.; Yoon, G.; Cheon, S. H. Bioorg. Med. Chem. Lett. 2011, 21, 3755.
[17] Ge, H. X.; Wang, L. C. Prog. Pharm. Sci. 2005, 29, 310 (in Chinese).
(葛海霞, 王礼琛, 药学学报, 2005, 29, 310.)
[18] Patil, V.; Tilekar, K.; Mehendale-Munj, S.; Mohan, R.; Ramaa, C. S. Eur. J. Med. Chem. 2010, 49, 4539.
[19] Meng, F. L.; Zheng, C. J.; Li, Y. J.; Sun, L. P.; Liu, X. K.; Zhang, T. Y.; Piao, H. R. Chin. J. Org. Chem. 2012, 32, 183 (in Chinese).
(孟凡领, 郑昌吉, 李因晶, 孙良鹏, 刘学坤, 张天一, 朴虎日, 有机化学, 2012, 32, 183.)
[20] (a) Shi, L.; Yu, H. P.; Zhou, Y. Y.; Du, J. Q.; Shen, Q.; Li, J. Y.; Li, J. Acta Pharmacol. Sin. 2008, 29, 278.
(b) Zhang, W.; Hong, D.; Zhou, Y. Y.; Zhang, Y. N.; Shen, Q.; Li, J. Y.; Hu, L. H.; Li, J. Biochim. Biophys. Acta 2006, 1760, 1505.
/
| 〈 |
|
〉 |