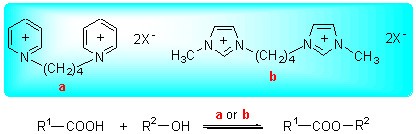
合成了双-(3-甲基-1-咪唑)亚丁基双对甲苯磺酸盐(Im-PTSA)、双-(3-甲基-1-咪唑)亚丁基双硫酸氢盐(Im-HSO4)、双-(1-吡啶)亚丁基双对甲苯磺酸盐(Py-PTSA)、双-(1-吡啶)亚丁基双硫酸氢盐(Py-HSO4)等4种功能化双核离子液体. 分别采用红外光谱(FT-IR)、核磁共振氢谱(1H NMR)对合成的离子液体进行结构分析; 采用热重(TG)测试了离子液体的热稳定性; 此外, 考察了离子液体的酸性和溶解性. 以丁二酸和乙醇的酯化反应考察了4种离子液体的催化活性, 结果表明: 当n(C4H6O4):n(C2H5OH)=1:3, 催化剂Im-PTSA量占总质量的1.90%, 反应温度70 ℃, 反应时间2.5 h, 酯收率可达93.6%, 选择性达100%, 且离子液体经真空干燥重复使用8次, 催化活性没有明显降低. 以奥氏体316 L不锈钢为试样, 考察了双核功能化离子液体的腐蚀性, 与浓硫酸进行对比, 其对钢试样的腐蚀率不到浓硫酸的1/10. 以双-(3-甲基-1-咪唑)亚丁基双对甲苯磺酸盐(Im-PTSA)为催化剂, 考察了一元有机酸和二元有机酸与系列醇的酯化反应, 均获得较高的酯收率和选择性, 反应结束后产品与催化剂自动分层, 简化了分离, 有望成为一种具有发展潜力的酯化催化剂.
A series of functional binuclear ionic liquids based on bis-(3-methyl-1-imidazole)butylidene double P-toluene sulfonic acid salt (Im-PTSA), bis-(3-methyl-1-imidazole)butylidene double bisulfate (Im-HSO4), bis-(1-pyridine)butylidene double p-toluene sulfonic acid salt (Py-PTSA), bis-(1-pyridine)butylidene double bisulfate (Py-HSO4) were synthesized by a two-step proceeding and their structures were characterized by FT-IR and 1H NMR spectra. Their thermal stabilities were characterized by TG. In addition, the acidity and solubility of functional binuclear ionic liquids were also studied. The catalytic activity of the binuclear ionic liquids for the esterification of succinic acid with ethanol was measured. The results show that under the optimized conditions of n(succinic acid):n(ethanol)=1:3, catalyst used dosage 1.90% (wt), 70 ℃ and 2.5 h, the yield of diethyl succinate reached 93.6% and the selectivity was near up to 100%. Im-PTSA was reused at least 8 times without significant decrease in activity after drying under vacuum. Austenitic stainless steel 316L was used for conducting the corrosion test under the above esterificaion condition, the corrosion rates of the steel plates dipped in the systems with these ionic liquids were less than one tenth of that with sulfuric acid. Fischer esterification of monocarboxylic acids and dicarboxylic acids with different alcohols was observed on using Im-PTSA as catalyst which gave high product yield and selectivity. Use of such a reaction catalyst should be appreciated for its convenient separation.
[1] Joseph, T.; Sahoo, S.; Halligudi, S. B. J. Mol. Catal. A: Chem. 2005, 234, 107.
[2] Xie, C.; Li, H.; Li, L.; Yu, S.; Liu, F. J. Hazard. Mater. 2008, 151, 847.
[3] Li, X.; Eli, W. J. Mol. Catal. A: Chem. 2008, 279, 159.
[4] Li, X.; Lin, Q.; Ma, L. Ultrason. Sonochem. 2010, 17, 752.
[5] Sunitha, S.; Kanjilal, S.; Reddy, P. S.; Prasad, R. B. N. Tetrahedron Lett. 2007, 48, 6962.
[6] Janus, E.; Goc-Maciejewska, I.; Lozynski, M.; Pernak, J. Tetrahedron Lett. 2006, 47, 4079.
[7] Durand, J.; Teuma, E.; Gómez, M. C. R. Chim. 2007, 10, 152.
[8] Cai, S.; Wang, S. Chin. J. Chem. Eng. 2011, 19, 57.
[9] Zhao, Y.; Li, Z.; Xia, C. Chin. J. Catal. 2011, 32, 440.
[10] Deng, Y.; Shi, F.; Beng, J.; Qiao, K. J. Mol. Catal. A: Chem. 2001, 165, 33.
[11] Fraga-Dubreuil, J.; Bourahla, K.; Rahmouni, M.; Bazureau, J. P.; Hamelin, J. Catal. Commun. 2002, 3, 185.
[12] Cole, A. C.; Jensen, J. L. J. Am. Chem. Soc. 2002, 124, 5962.
[13] Kou, Y.; Yang, Y.-L. Petrochem. Technol. 2004, 33, 297 (in Chinese).
(寇元, 杨雅立, 石油化工, 2004, 33, 297.)
[14] Zhang, S.-J.; Lv, X.-M. Ionic Liquid—From Basic Research to Industrial Applications, Science Press, Beijing, 2006, p. 71 (in Chinese).
(张锁江, 吕兴梅, 离子液体——从基础研究到工业应用, 科学出版社, 北京, 2006, p. 71.)
[15] Lv, X.-M. M.S. Thesis, Nanjing University, Nanjing, 2011 (in Chinese).
(吕学铭, 硕士论文, 南京大学, 南京, 2011.)
[16] Bermúdez, M. D.; Jiménez, A. E.; Martínez-Nicolás, G. Appl. Surf. Sci. 2007, 253, 7295.
[17] Ganeshpure, P. A.; George, G.; Das, J. J. Mol. Catal. A: Chem. 2008, 279, 182.
[18] Liang, J.-H.; Xu, Y.; Ren, X.-Q.; Jiang, M.; Li, Z.-J. Acta PetroleI Sin. 2011, 27, 482 (in Chinese).
(梁金花, 徐玥, 任晓乾, 姜岷, 李振江, 石油学报, 2011, 27, 482.)
[19] Huang, B.-H.; Li, Z.-J.; Shi, N.; Xu, X.-L.; Zhang, K.; Fang, Y.-X. Chin. J. Org. Chem. 2009, 29, 770 (in Chinese).
(黄宝华, 黎子进, 史娜, 徐效陵, 张焜, 方岩雄, 有机化学, 2009, 29, 770.)



